Schiff Reagent, McManus
Tech Memo 1: Schiff Reagent, McManus for Periodic Acid Schiff (PAS) Stain
SOLUTION:
| 125 ml | 250 ml | 500 ml | 1 Liter | 4 Liters | |
| Schiff Reagent, McManus | Part 1371A | Part 1371E | Part 1371B | Part 1371C | Part 1371D |
Additionally Needed For Periodic Acid Schiff (PAS) Stain:
| Periodic Acid Schiff (PAS) Glycogen Control Slides | Part 4540 |
| Periodic Acid 0.5%, Aqueous | Part 13308 |
| Hematoxylin Stain, Harris Modified | Part 1201 |
| Acid Alcohol 1% | Part 10011 |
| Lithium Carbonate, Saturated Aqueous | Part 12215 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Schiff Reagent, McManus a crucial element in the Periodic Acid Schiff (PAS) Stain and for staining glycoproteins, epithelial mucins, basement membranes and fungal cell walls. Schiff Reagent, McManus is used in a variety of staining procedures that include:
-
- PAS with and without diastase
- Alcian Blue/PAS for mucins
- PAS/Light Green for Fungus
- Feulgen Reaction for demonstration of DNA
Schiff Reagent, McManus is a stable and reliable product that is conveniently stored at room temperature for ready-to-use staining. Although initially colorless, Schiff Reagent, McManus when in direct contact with skin, clothing, countertops, floors and other surfaces, will react and leave a bright magenta stain that is difficult to remove.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions manufactured by Newcomer Supply, Inc.
Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below.
PAS STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place in Periodic Acid 0.5%, Aqueous (Part 13308) for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Schiff Reagent, McManus for 20 minutes.
- See Procedure Notes #3, #4 and #5.
- Wash slides in lukewarm tap water for 5-10 minutes.
- Stain with Hematoxylin Stain, Harris Modified (Part 1201), 1-5 minutes, depending on preference of nuclear stain intensity.
- Wash in tap water for 2-3 minutes.
- Differentiate in Acid Alcohol 1% (Part 10011); 1-2 quick dips.
- Wash in tap water for 1 minute.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215); 3-4 dips.
- Wash in several changes of tap water; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
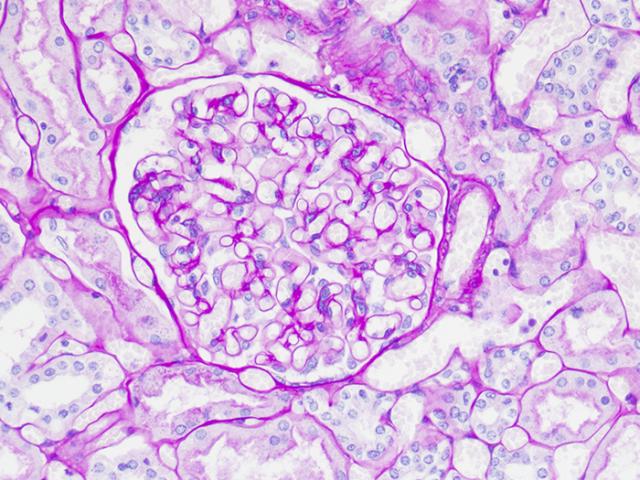
RESULTS:
| Glycogen | Magenta |
| Acid & neutral epithelial mucin | Magenta |
| Fungal cell walls | Red to purple |
| Basement membranes | Red to purple |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Once used, do not pour Schiff Reagent, McManus back into the original bottle and/or mix with fresh solution.
- To test quality of Schiff Reagent, McManus reactivity;
- All glassware/plasticware containing Schiff Reagent, McManus should be thoroughly rinsed with running tap water to remove residual stain prior to standard glassware cleaning.
- Digestion steps can be employed in the PAS procedure for further identification of mucosubstances.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 168-174, 180.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.137-141.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 164-168, 245.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo 2: Schiff Reagent, McManus for Fungus, PAS/Light Green
SOLUTION:
| 125 ml | 250 ml | 500 ml | 1 Liter | 4 Liters | |
| Schiff Reagent, McManus | Part 1371A | Part 1371E | Part 1371B | Part 1371C | Part 1371D |
Additionally Needed For Fungus Stain, PAS/Light Green:
| Fungus, PAS, Aspergillus sp., Artificial Control Slides OR Fungus, PAS, Candida sp., Artificial Control Slides |
Part 4232 OR Part 4233 |
| Periodic Acid 0.5%, Aqueous | Part 13308 |
| Light Green SF Yellowish Stain 0.1%, Aqueous | Part 12203 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Schiff Reagent, McManus, a crucial element in the Fungus, PAS/Light Green procedure, is used for staining fungus and glycogen in tissue sections.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin section cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below.
FUNGUS, PAS/LIGHT GREEN STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place in Periodic Acid 0.5%, Aqueous (Part 13308) for 5 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Drain slides of excess water and stain in Schiff Reagent, McManus for 20 minutes.
- Wash gently in lukewarm tap water for 10 minutes to allow pink color to develop.
- Counterstain in Light Green SF Yellowish Stain 0.1%, Aqueous (Part 12203) for 5 seconds.
- See Procedure Note #3.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Fungal cell walls and glycogen | Red to magenta |
| Background | Pale green |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Increase or decrease staining time in Light Green SF Yellowish Stain 0.1%, Aqueous for preference of counterstain intensity.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 321-323.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245.
- Modifications developed by Newcomer Supply Laboratory.


.jpg)
