Safranin O Stain 1%, Aqueous
(use: Cartilage Staining.)
SOLUTION:
| 125 ml | 500 ml | |
| Safranin O Stain 1%, Aqueous | Part 1350A | Part 1350B |
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Hematoxylin Stain Set, Weigert Iron | Part 1409 |
| Fast Green Stain 2.5%, Aqueous | Part 10852 |
| Acetic Acid 1%, Aqueous | Part 10012 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
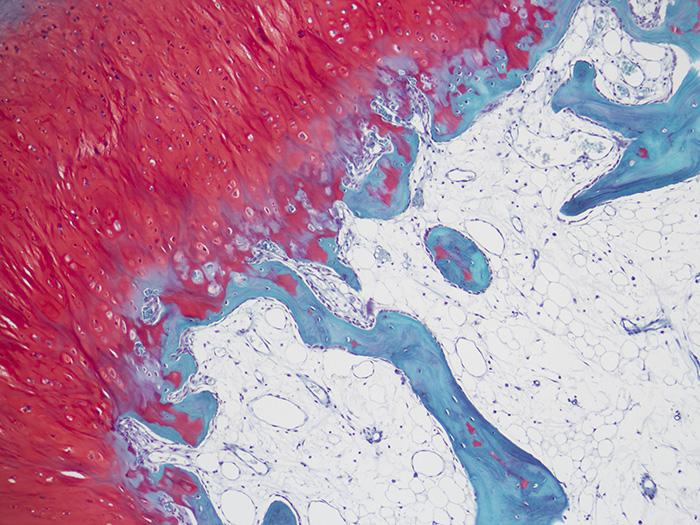
Newcomer Supply Safranin O Stain for Cartilage procedure is used to stain cartilage, mucin, and mast cell granules in tissue sections and can assist in demonstrating articular cartilage loss in arthritic and other articular disease processes.
Safranin O is a basic dye that stains growth plate cartilage and articular cartilage (proteoglycans, chondrocytes and type II collagen) varying shades of red. The intensity of Safranin O staining is proportional to the proteoglycan content in the cartilage tissue. Fast Green counterstains the non-collagen sites and provides a clear contrast to the Safranin O staining.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Decalcified paraffin sections cut at 4 microns
-
-
- See Procedure Note #1.
-
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #2 and #3.
-
- Wash well in running tap water; rinse in distilled water.
- Prepare fresh Weigert Iron Hematoxylin (Part 1409); mix well.
-
- Solution A: Ferric Chloride, Acidfied 20 ml
- Solution B: Hematoxylin 1%, Alcoholic 20 ml
-
- Stain in fresh Weigert Iron Hematoxylin for 10 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
-
- See Procedure Note #4.
-
- Prepare 0.25% Fast Green Stain, Aqueous; combine and mix well.
-
- Fast Green Stain 2.5%, Aqueous (Part 10852) 5 ml
- Distilled Water 45 ml
-
- Stain in 0.25% Fast Green Stain, Aqueous for 5 minutes.
- Rinse directly in Acetic Acid 1%, Aqueous (Part 10012); 10-15 seconds.
- Place directly in Safranin O Stain 1%, Aqueous for 5 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Cartilage | Red to orange |
| Mucin and mast cell granules | Red to orange |
| Bone, connective tissue and cytoplasm | Green |
| Nuclei | Black |
PROCEDURE NOTES:
-
- Fixatives and decalcifying agents such as EDTA, nitric acid and hydrochloric acid can work to extract proteoglycans and result in weak Safranin O cartilage staining.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If Weigert Iron Hematoxylin is not completely washed from sections, nuclear and cytoplasmic staining will be compromised.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 346-347.
- Callis, Gayle and Diane Sterchi. “Decalcification of Bone: Literature Review and Practical Study of Various Decalcifying Agents, Methods, and Their Effects on Bone Histology.” The Journal of Histotechnology 1 (1998): 49-58.
- Chevrier, Anik, Evgeny Rossomacha, Michael D. Buschmann, and Caroline D. Hoemann. “Optimization of Histoprocessing Methods to Detect Glycosaminoglycan, Collagen Type II, and Collagen Type I in Decalcified Rabbit Osteochondral Sections.” The Journal of Histotechnology 3 (2005): 165-175.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 256.
- Modifications developed by Newcomer Supply Laboratory.