Newly Blue Stain Set
Hematoxylin Alternative Nuclear Stain
-
-
- Regressive Stain
-
Solution A: Celestine Blue Stain 1%, Aqueous 250ml
Solution B: Ferric Ammonium Sulfate 4%, Aqueous 250ml
Samples Available
SET INCLUDES:
| Part 1258A | ||
| Solution A: | Celestine Blue Stain 1%, Aqueous | 250ml |
| Solution B: | Ferric Ammonium Sulfate 4%, Aqueous | 250 ml |
Additionally Needed For H&E Staining:
| Hematoxylin and Eosin (H&E) Control Slides | Part 4278 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Acid Alcohol 1% | Part 10011 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Eosin Y Working Solution | Part 1072 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
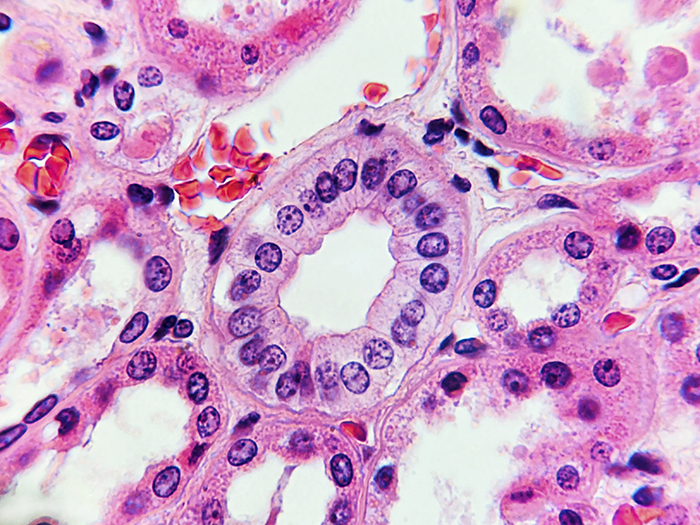
Newcomer Supply Newly Blue Stain Set provides a synthetic nuclear stain (hematoxylin substitute), that is indistinguishable from standard hematoxylin staining results. Newly Blue nuclear staining is crisp with well delineated purple to blue nuclei and displays a clear contrast to cytoplasmic stains for precise cellular interpretation.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Staining Sets are designed to be used with Coplin jar filled to 40 ml following the provided staining procedure.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Prepare Newly Blue Working Solution and mix well:
-
- Solution A: Celestine Blue Stain 1%, Aqueous 20 ml
- Solution B: Ferric Ammonium Sulfate 4%, Aqueous 20 ml
- Filter before use.
- See Procedure Notes #1 & #2.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #3 and #4.
-
- Stain with Newly Blue Working Solution for 4 minutes.
- Wash well in three changes of tap water.
- Differentiate quickly in Acid Alcohol 1% (Part 10011).
-
- Nuclei should be distinct; background light to colorless.
-
- Rinse well in three changes of tap water.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- Wash in three changes of tap water; rinse in distilled water.
- Drain excess water; proceed to 70% alcohol for 10 dips.
- Counterstain in Eosin Y Working Solution (Part 1072) or prepared Eosin-Phloxine Working Solution (Part 1082) for 30 seconds to 3 minutes, depending on preference of intensity.
- Dehydrate in two changes of 95% ethyl alcohol for 1 minute each and two changes of 100% ethyl alcohol, 10 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Nuclei | Blue |
| Cytoplasm and other tissue elements | Various shades of pink |
PROCEDURE NOTES:
-
- Newly Blue Working Solution is stable for up to 5 days.
- Blot off any surface sheen that may develop prior to use.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 115-116.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 148-150.
- Modifications developed by Newcomer Supply Laboratory.