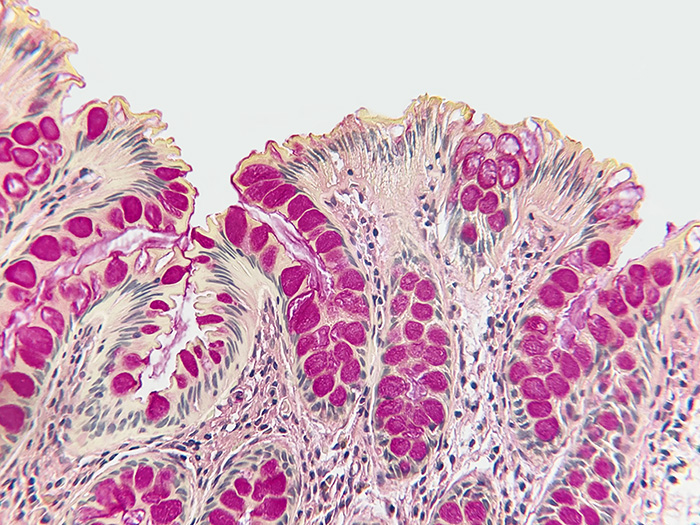
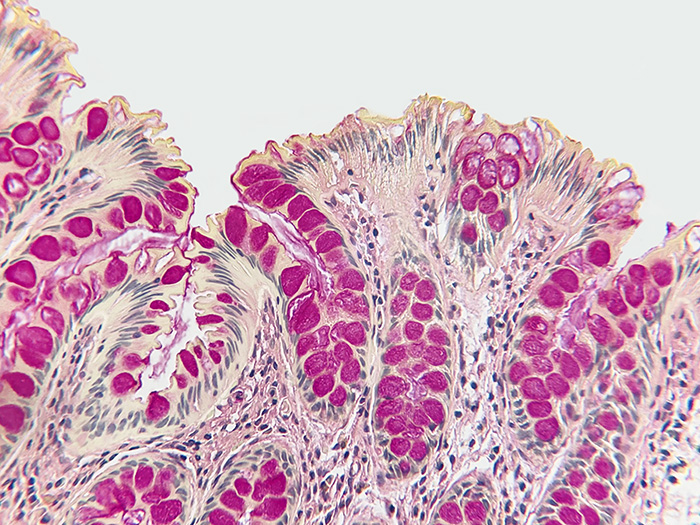
Mucin Mucicarmine
|
Validation Stain: Mucin Mucicarmine
|
PRODUCT SPECIFICATIONS:
Tissue: Positive staining colon.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Mayer Mucicarmine quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Mucin, Mayer Mucicarmine Stain Kit: | Part 9151A/B | Individual Stain Solution | |
| Solution A: | Ferric Chloride, Aqueous | 125/250 ml | Part 1409 |
| Solution B: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution C: | Mucicarmine Stock Stain, Mayer | 125/125 ml | Part 1250 |
| Solution D: | Metanil Yellow Stain, Aqueous | 250/500 ml | Part 12235 |
APPLICATION:
Newcomer Supply Mucin Mucicarmine, Control Slides are for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin).
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Prepare fresh Weigert Iron Hematoxylin Working Solution directly before use; combine and mix well:
- Solution A: Ferric Chloride, Aqueous 20 ml
- Solution B: Hematoxylin 1%, Alcoholic 20 ml
- Stain in fresh Weigert Iron Hematoxylin Working Solution for 7 minutes.
- Rinse in running tap water for 10 minutes.
- Prepare fresh Mayer Mucicarmine Working Solution; combine and mix well:
- Solution C: Mucicarmine Stock Stain, Mayer 10 ml
- Tap Water (do not use distilled water) 30 ml
- Stain in fresh Mayer Mucicarmine Working Solution for 60 minutes or longer if a more intense stain is desired.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing fresh Mayer Mucicarmine Working Solution and microwave at 70°C for 10 minutes.
- Rinse in several changes of tap water.
- Counterstain in Solution D: Metanil Yellow Stain, Aqueous for 30 to 60 seconds.
- Dehydrate quickly through 95% and 100% ethyl alcohols. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Deep rose to red |
| Nuclei | Black |
| Other tissue elements | Yellow |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 174-175.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 142-144.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 168-169.
- Modifications developed by Newcomer Supply Laboratory.