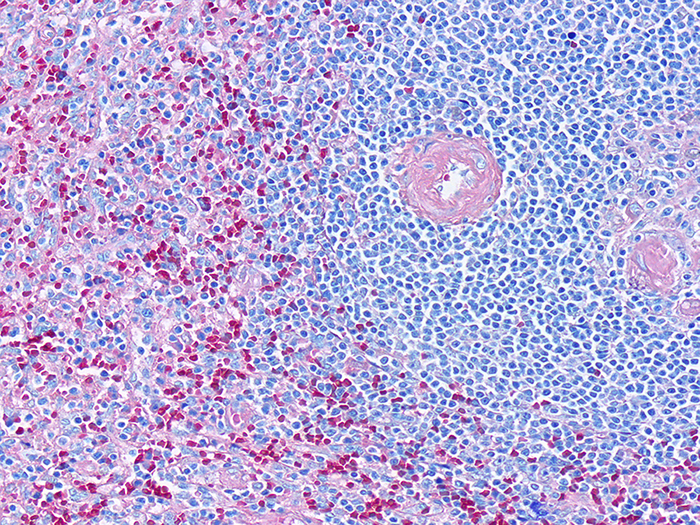
Giemsa
|
Validation Stain: May-Grunwald
Other Applicable Stains: Wolbach Giemsa and Wright Giemsa
|
Representing the general hematopoietic population.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: May-Grunwald Giemsa quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With May-Grunwald Giemsa Stain: | Individual Stain Solution |
| Jenner Stock Stain | Part 1210 |
| Giemsa Stock Stain, Wolbach | Part 1121 |
| Alcohol, Methanol Anhydrous, ACS | Part 12236 |
| Acetic Acid 1%, Aqueous | Part 10012 |
APPLICATION:
Newcomer Supply Giemsa Control Slides are for the positive histochemical and differential staining of hematopoietic tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Rinse in two changes of Alcohol, Methanol Anhydrous, ACS (Part 12236); 3 minutes each.
- Prepare fresh Working Jenner Stain Solution just prior to use; combine and mix well.
- Distilled Water 20 ml
- Jenner Stock Stain 20 ml
- Place slides in fresh Working Jenner Stain Solution for 6 minutes.
- Prepare fresh Working Giemsa Stain Solution just prior to use; combine and mix well.
- Distilled Water 47 ml
- Giemsa Stock Stain, Wolbach 3 ml
- Place slides directly into fresh Working Giemsa Stain Solution for 45 minutes.
- Rinse quickly in distilled water.
- Differentiate each slide individually in Acetic Acid 1%, Aqueous (Part 10012); 6-10 dips.
- Check microscopically for well differentiated nuclei.
- Rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Nuclei | Blue/violet |
| Cytoplasm | Pink/rose to lighter blue shades |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The color range of the stained cells may vary depending upon fixation and degree of differentiation.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 121-122.
- Shapiro, Stanley H., and Hilda Laufer. “Observations on Fixation and Staining of Bone Marrow Biopsies.” The Journal of Histotechnology 11.3 (1988): 145-47.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 157.
- Modifications developed by Newcomer Supply Laboratory.