Fite, Leprosy, Animal
|
Validation Stain: Fite
|
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: AFB, Fite quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With AFB, Fite Stain Kit: | Part 91013A | Individual Stain Solution | |
| Solution A: | Xylene/Peanut Oil, 2:1 | 500 ml | Part 1449 |
| Solution B: | Carbol Fuchsin Stain, Ziehl-Neelsen | 250 ml | Part 1030 |
| Solution C: | Acid Alcohol 1% | 250 ml | Part 10011 |
| Solution D: | Light Green SF Yellowish 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
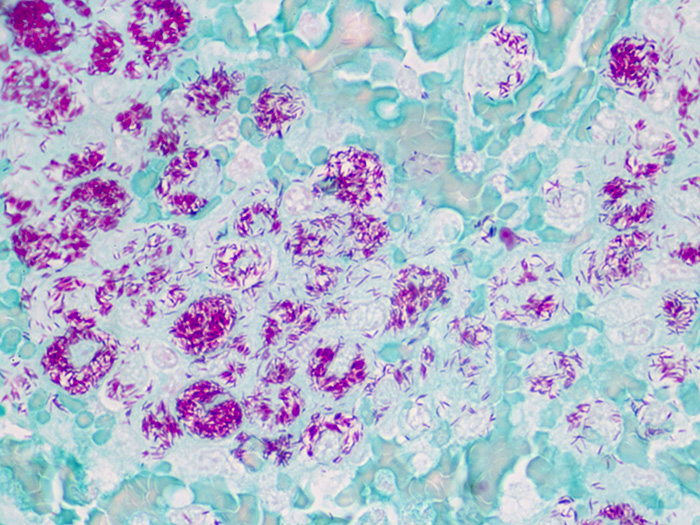
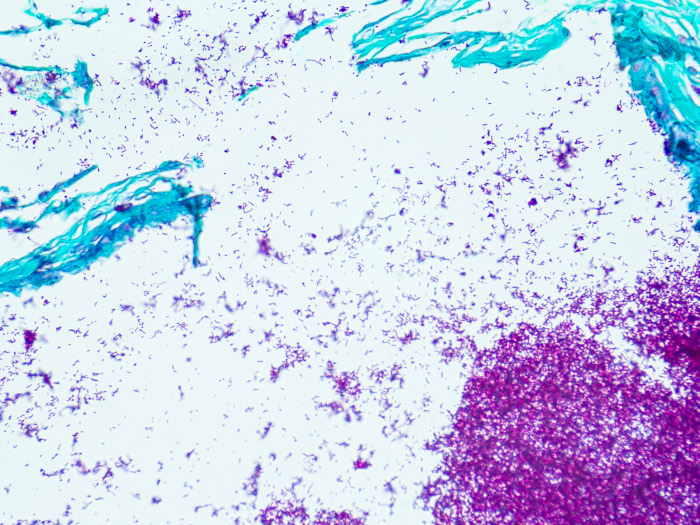
Newcomer Supply Fite, Leprosy, Animal Control Slides are for the positive histochemical staining of Mycobacterium leprae, the causative agent of leprosy.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen with high quality filter paper.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 10 minutes each.
-
- See Procedure Note #1.
-
- Drain slides, wipe off excess oil, and blot to opacity taking care to remove residual oil.
-
- See Procedure Note #2.
-
- Stain in freshly filtered Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature.
- Rinse well in distilled water.
- Differentiate slides individually in Solution C: Acid Alcohol 1% until sections are light pink; 5-10 dips.
- Rinse well in distilled water.
- Counterstain in Solution D: Light Green SF Yellowish 0.1%, Aqueous; 5-10 dips.
- Rinse in distilled water.
- Blot excess water from slide and air-dry or oven-dry completely.
- Dip dried slides in xylene and coverslip with a compatible mounting medium.
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 10 minutes each.
RESULTS:
| Mycobacterium leprae | Red |
| Other tissue elements | Green |
PROCEDURE NOTES:
-
- Acid-fastness of leprosy organisms is enhanced when the waxy capsule is protected by the mixture of xylene/peanut oil and avoidance of dehydrating solutions.
- It is important to blot well, residual oil may produce staining artifact.
- If using a xylene substitute, follow manufacturer’s recommendation for coverslipping step.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-instructional Text. 5th ed. Chicago: ASCP Press, 2020. 215-216.
- Fite, George, P.J. Cambre and M.H. Turner. “Procedure for Demonstrating Lepra Bacilli in Paraffin Sections”. Archives of Pathology 43 (1947). 624-625.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.