Colloidal Iron, Multi-Tissue
|
Validation Stain: Muller Mowry
|
Why use a Colloidal Iron Multi-Tissue Control Slide?
When a single control tissue containing a mucosubstance (small intestine) used in the colloidal iron stain produces negative results, what do those negative results mean? Did the colloidal iron solution not react or did the potassium ferrocyanide (Prussian Blue solution) step cause the negative reaction? Colloidal Iron Multi-Tissue Control Slides answers those questions.
How does the Colloidal Multi-Tissue Control Slide work?
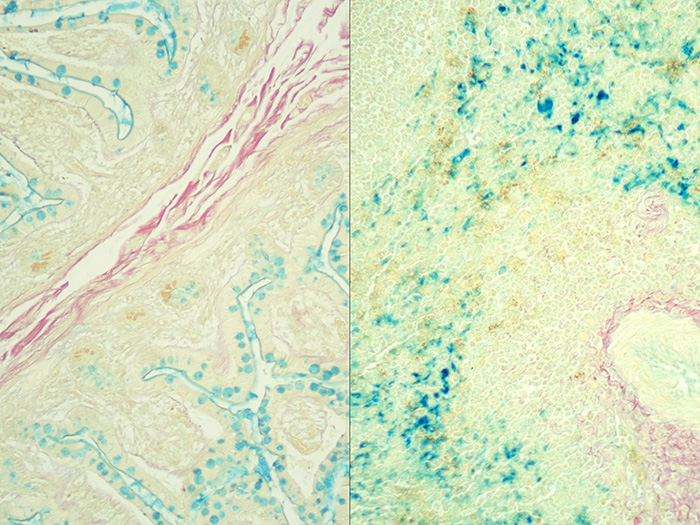
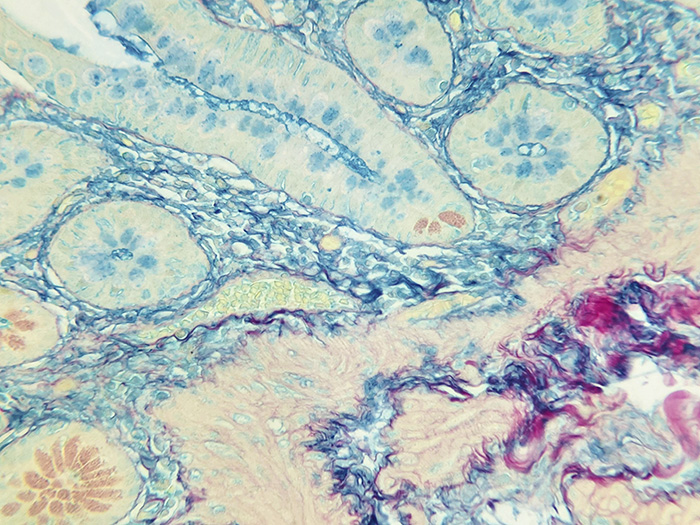
Small Intestine Tissue acts as the Colloidal Iron Solution check. When small intestine tissue exposed to a colloidal iron solution is in an acidic environment, ferric ions are absorbed by acid mucosubstances.
Spleen Tissue acts as the potassium ferrocyanide (Prussian Blue solution) check. Spleen tissue contains a substantial amount of iron and stains in the presence of Prussian Blue.
What Colloidal Iron Multi-Tissue Control Slide results mean:
| Small Intestine | Spleen | Reaction |
| POSITIVE | POSITIVE | All reaction steps have occured |
| NEGATIVE | POSITIVE | Colloidal Iron is source of negative reaction |
| NEGATIVE | NEGATIVE | Prussian Blue is source of negative reaction |
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine and positive iron staining animal spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Müller-Mowry Colloidal Iron quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Colloidal Iron, Müller-Mowry Stain Kit: | Part 9110A | Individual Stain Solution | |
| Solution A: | Acetic Acid 12%, Aqueous | 1000 ml | |
| Solution B: | Colloidal Iron Stock | 125 ml | Part 10365 |
| Solution C: | Acetic Acid, Glacial, ACS | 50 ml | Part 10010 |
| Solution D: | Potassium Ferrocyanide 2%, Aqueous | 125 ml | |
| Solution E: | Hydrochloric Acid 2%, Aqueous | 125 ml | |
| Solution F: | Van Gieson Stain | 250 ml | Part 1404 |
APPLICATION:
Newcomer Supply Colloidal Iron, Multi-Tissue Control Slides, use a combination of tissue sources for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin) and stromal (mesenchymal) mucin in small intestine and ferric iron in spleen.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Acid clean glassware prior to use to avoid residual iron staining.
- See Procedure Note #1.
- Prepare Colloidal Iron Working Solution; combine and mix well.
- Solution B: Colloidal Iron Stock 20 ml
- Solution C: Acetic Acid, Glacial ACS 5 ml
- Distilled Water 15 ml
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #2 and #3.
- Place in Solution A: Acetic Acid 12%, Aqueous for 30 seconds.
- Drain Slides. Do not rinse.
- Place in Colloidal Iron Working Solution (Step #2) for 30 minutes.
- Rinse in three changes of Solution A: Acetic Acid 12%, Aqueous; 3 minutes each.
- Prepare fresh Ferrocyanide-Hydrochloric Acid Solution directly before use; combine and mix well.
- Solution D: Potassium Ferrocyanide 2%, Aqueous 20 ml
- Solution E: Hydrochloric Acid 2%, Aqueous 20 ml
- Place in Ferrocyanide-Hydrochloric Acid Solution for 15 minutes.
- Wash in running tap water for 1-5 minutes.
- Counterstain in Solution F: Van Gieson Stain for 3-5 minutes.
- Proceed directly to dehydration step without rinsing.
- Dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Blue |
| Stromal mucin | Blue |
| Ferric iron deposits | Bright blue |
| Collagen | Red |
| Muscle and cytoplasm | Yellow |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Nuclear Fast Red Stain, Kernechtrot (Part 1255) can be used as an alternative counterstain.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 175-176.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 151-153.
- Rekhtman, Natasha, and Justin Bishop. Quick Reference Handbook for Surgical Pathologists. Berlin: Springer, 2011. 69.
- Modifications developed by Newcomer Supply Laboratory.