Tartrazine Stain 1.5%, Aqueous
SOLUTION:
| 500 ml | 1 Liter | |
| Tartrazine Stain 1.5%, Aqueous | Part 14015A | Part 14015B |
Additionally Needed:
| Gram, Multi-Tissue, Artificial Control Slides OR Gram+ & Gram- Bacteria, Artificial Control Slides |
Part 4256 OR Part 4255 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Crystal Violet Stain 1%, Aqueous, Brown-Hopps | Part 1041 |
| Iodine, Gram, Aqueous | Part 1140 |
| Acetone, ACS | Part 10014 |
| Basic Fuchsin Stain 0.25%, Aqueous | Part 1011 |
| Gallego Solution | Part 1098 |
| Acetone-Xylene 1:1 | Part 10015 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
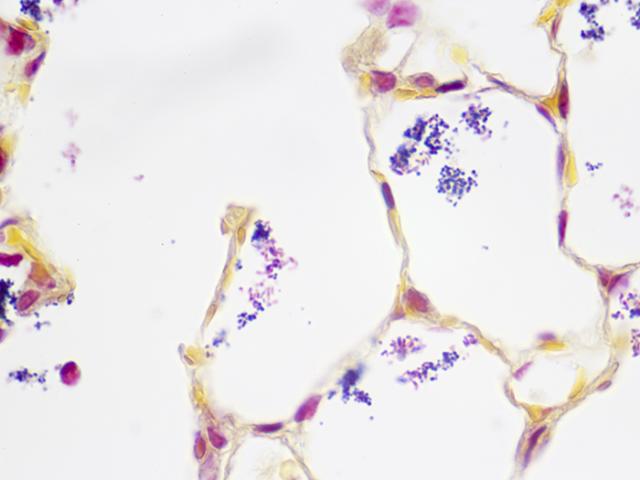
Newcomer Supply Brown-Hopps Modified Gram Stain with Tartrazine is a modification of the original Gram stain technique. Tartrazine, a synthetic water soluble, lemon yellow colored azo dye, provides a safe alternative to picric acid stains. Tartrazine Stain 1.5%, Aqueous provides yellow background staining, replacing the picric acid-acetone counterstain and the disposable issues associated with picric acid.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Stain in Crystal Violet Stain 1%, Aqueous, Brown-Hopps (Part 1041) for 2 minutes.
- Rinse well in distilled water.
- Mordant in Iodine, Gram, Aqueous (Part 1140) for 5 minutes.
- Rinse well in distilled water.
- Blot excess water from slide; decolorize one slide at a time in Acetone (Part 10014) until the blue stops running, 1-2 dips.
- Sections should be very light gray in color.
- Quickly rinse in running tap water.
- Place in Basic Fuchsin Stain 0.25% Aqueous (Part 1011) for 5 minutes.
- Rinse well in running tap water.
- Differentiate sections in Gallego Solution (Part 1098) for 5 minutes.
- Rinse in running tap water. Blot water off slide(s) but not to dryness.
- Proceed with Steps #13 to #16 one slide at a time.
- Place in Tartrazine Stain 1.5%, Aqueous for 1 minute.
- Rinse well in running tap water.
- Dip in Acetone, 1-2 quick dips.
- Dip in Acetone-Xylene 1:1 (Part 10015) for 5 dips.
- Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Gram-positive bacteria | Blue |
| Gram-negative bacteria | Red |
| Nuclei | Red |
| Background tissue | Yellow |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Brown, Robert C., and Howard C. Hopps. “Staining of Bacteria in Tissue Sections: A Reliable Gram Stain Method.” American Journal of Clinical Pathology 60.2 (1973): 234-240.
- Dapson, Janet Crookham, and Richard Dapson. Hazardous Materials in the Histopathology Laboratory: Regulations, Risks, Handling, and Disposal. 4th ed. Battle Creek, MI: Anatech, 2005. 150, 182, 266.
- Mercado, Gene. “Modifying the Modification: How to Redden Shy Gram Negatives.” The Journal of Histotechnology 25.2 (2002): 115-116.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 235.
- Modifications developed by Newcomer Supply Laboratory.