Gram, Multi-Tissue, Artificial
|
Validation Stain: Gram, Brown-Brenn
Other Applicable Stains: Gram, Brown-Hopps and Gram-Twort
|
(Not in human tissue.) Organisms artificially introduced into a rat organ (e.g., lung) through a non-infective process.
PRODUCT SPECIFICATIONS:
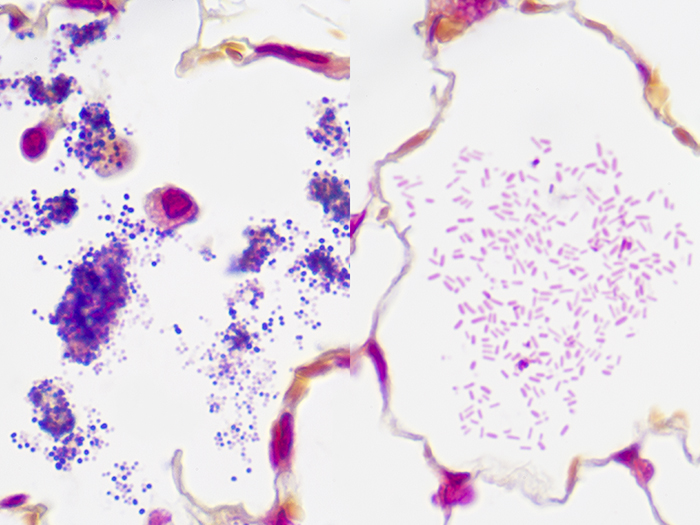
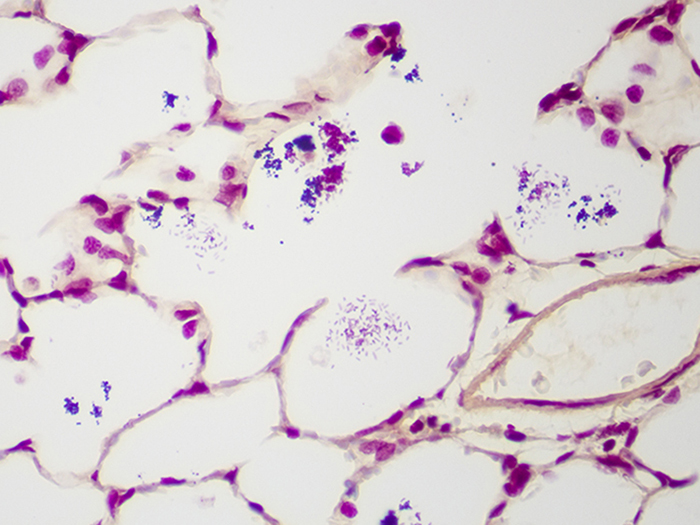
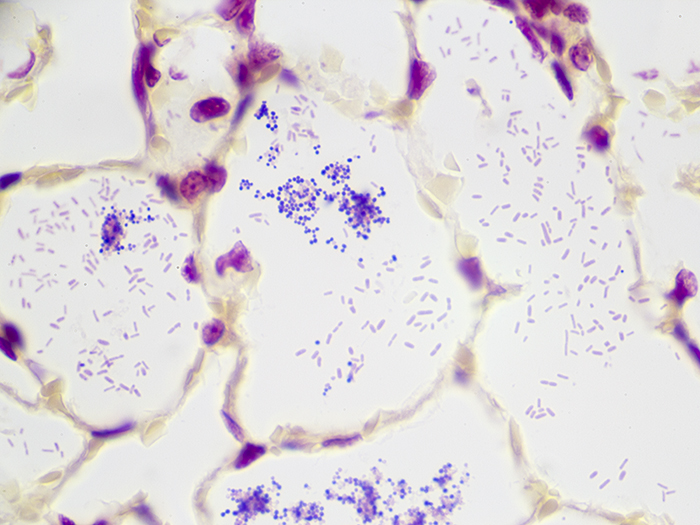
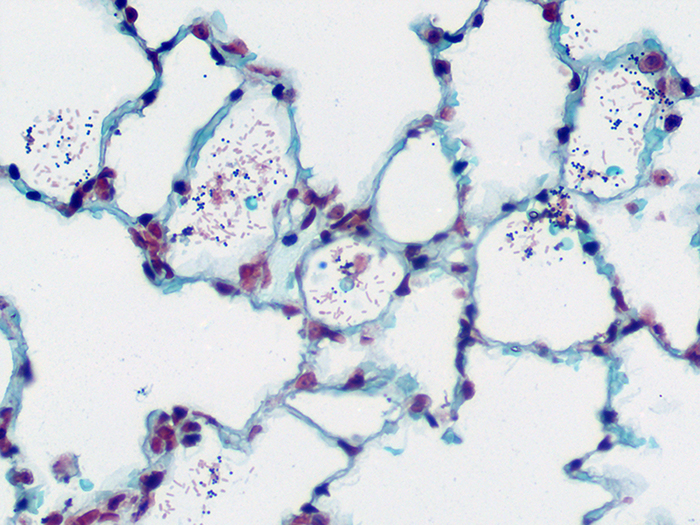
Tissue: Gram positive staining rat lung and gram negative staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Brown-Brenn quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Gram, Brown-Brenn, Stain Kit: | Part 9123A | Individual Stain Solution | |
| Solution A: | Crystal Violet-Oxalate Stain, Alcoholic | 250 ml | Part 10422 |
| Solution B: | Iodine, Gram, Aqueous | 250 ml | Part 1140 |
| Solution C: | Acetone-Alcohol 1:1 | 250 ml | Part 10016 |
| Solution D: | Basic Fuchsin Stain 0.25%, Aqueous | 250 ml | Part 1011 |
| Solution E: | Tartrazine Stain 0.25%. Acetic Aqueous | 250 ml | Part 14016 |
APPLICATION:
Newcomer Supply Gram, Multi-Tissue, Artificial Control Slides are for the positive histochemical staining of gram positive and gram negative bacteria in separate tissue sections. Escherichia coli and Staphylococcus aureus are used to produce the positive controls.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution A: Crystal Violet-Oxalate Stain, Alcoholic.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in freshly filtered Solution A: Crystal Violet-Oxalate Stain, Alcoholic (Step #2) for 1 minute.
- Rinse well in distilled water.
- Mordant in Solution B: Iodine, Gram, Aqueous for 1 minute.
- Rinse well in distilled water, removing excess iodine.
- Decolorize in Solution C: Acetone-Alcohol 1:1 until blue stops running; 7-10 dips.
- Rinse well in distilled water.
- Place in Solution D: Basic Fuchsin Stain 0.25%, Aqueous for 90 seconds.
- Rinse well in distilled water.
- Dip once in Solution C: Acetone-Alcohol 1:1.
- Counterstain in Solution E: Tartrazine Stain 0.25%, Acetic Aqueous for 5-15 seconds.
- Rinse well in distilled water.
- Dehydrate in two changes of 100% ethyl alcohol, 5 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
- Do not use 95% alcohol in the dehydration step.
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Gram negative bacteria | Red |
| Gram positive bacteria | Blue/violet |
| Background tissue | Yellow |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 312-313.
- Brown, J.H., and L. Brenn. “A Method for the Differential Staining of Gram Positive and Gram Negative Bacteria in Tissue Sections”. Bulletin of The Johns Hopkins2 (1931): 69-73.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 188-189.
- Modifications developed by Newcomer Supply Laboratory.