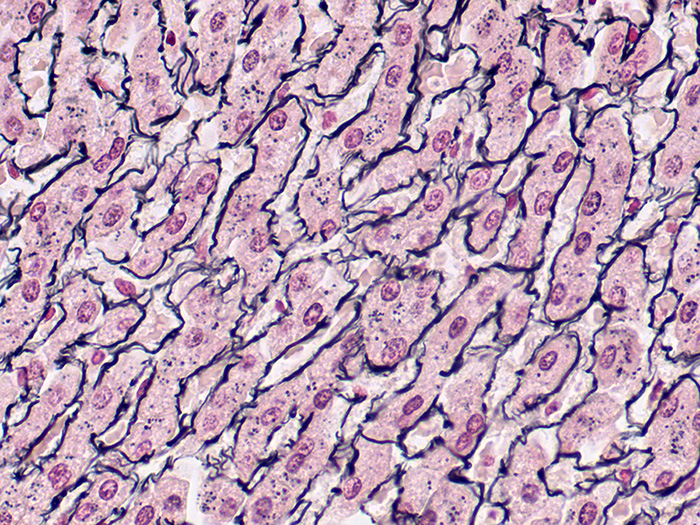
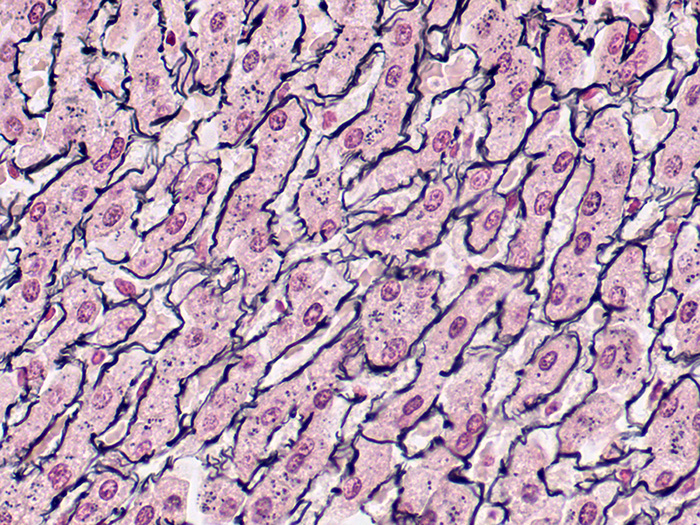
Reticulum, Gordon & Sweets Stain Kit
A silver stain for demonstration of reticular fibers. Useful in the differential diagnosis of certain types of tumors.
RETICULUM, GORDON & SWEETS STAIN KIT INCLUDES:
| Part 9168A | ||
| Solution A: | Potassium Permanganate 1%, Aqueous | 250 ml |
| Solution B: | Oxalic Acid 1%, Aqueous | 250 ml |
| Solution C: | Ferric Ammonium Sulfate 2.5%, Aqueous | 250 ml |
| Solution D: | Silver Nitrate 10%, Aqueous | 50 ml |
| Solution E: | Ammonium Hydroxide 28-30%, ACS | 50 ml |
| Solution F: | Sodium Hydroxide 3%, Aqueous | 50 ml |
| Solution G: | Formalin 10%, Aqueous | 250 ml |
| Solution H: | Gold Chloride 0.2%, Aqueous | 250 ml |
| Solution I: | Sodium Thiosulfate 5%, Aqueous | 250 ml |
| Solution J: | Nuclear Fast Red Stain, Kernechtrot | 250 ml |
| Disposable Plastic Pipettes (12) | ||
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed with this kit are two complimentary unstained positive control slides to be used for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Hydrochloric Acid 5%, Aqueous | Part 12086 (for acid cleaning glassware) |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Reticulum, Gordon & Sweets Stain Kit procedure is a silver staining method for demonstration of reticular fibers; regarded as specialized connective tissue fibers. This stain is useful in the differential diagnosis of certain types of tumors.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Prepare Silver Ammoniacal Working Solution in an acid cleaned Erlenmeyer flask. Save for Step #11.
- Solution D: Silver Nitrate 10%, Aqueous; 5 ml
- Add Solution E: Ammonium Hydroxide 28-30%, ACS drop by drop, mix with swirling motion until precipitate completely dissolves. Do not add excess Ammonium Hydroxide.
- Add 5 ml of Solution F: Sodium Hydroxide 3%, Aqueous.
- Re-dissolve precipitate with Solution E: Ammonium Hydroxide 28-30%, ACS drop by drop, mix with swirling motion until a faint silver/gray tinge remains. It is normal for trace precipitate to remain.
- If proceeded too far and solution is completely clear, add Solution D: Silver Nitrate 10%, Aqueous drop by drop, until one drop causes solution to reach silver/gray tinge.
- Bring solution volume to 50 ml with distilled water; filter.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Oxidize in Solution A: Potassium Permanganate 1%, Aqueous for 3 minutes.
- Wash in running tap water for 1 minute; rinse in distilled water.
- Bleach in Solution B: Oxalic Acid 1%, Aqueous for 2 minutes or until sections are colorless.
- Wash in running tap water for 1 minute; rinse in distilled water.
- Sensitize in Solution C: Ferric Ammonium Sulfate 2.5%, Aqueous; 10 to 15 minutes.
- Rinse in several changes of distilled water.
- Impregnate sections in filtered Silver Ammoniacal Working Solution (Step #3) for 2 minutes.
- Rinse well in running distilled water for 1 minute.
- See Procedure Note #5.
- Reduce in Solution G: Formalin 10%, Aqueous for 1 minute.
- Rinse in running tap water for 1 minute.
- Check control microscopically for black reticular fiber development.
- See Procedure Note #6.
- Tone in Solution H: Gold Chloride 0.2%, Aqueous for 1-2 minutes.
- Rinse well in distilled water.
- Place in Solution I: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash well in tap water for 1 minute; rinse in distilled water.
- Counterstain with Solution J: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #7.
- Quickly dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Reticular fibers | Black |
| Background | Red |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with silver solutions to prevent precipitation of silver salts. No metals of any kind should come in contact with silver solutions.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- This rinse step is critical for good reticulum demonstration. If rinsing is insufficient, excessive background staining may occur.
- If black reticular fibers are not evident or are lightly/poorly stained, return all slides to Silver Working Solution (Step #11) and repeat Steps 11-14 with the same timings.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 177-179
- Gordon, Harold, and Henry Sweets. “A Simple Method for the Silver Impregnation of Reticulum.” American Journal of Pathology 12.4 (1936): 545-552.
- Modifications developed by Newcomer Supply Laboratory.