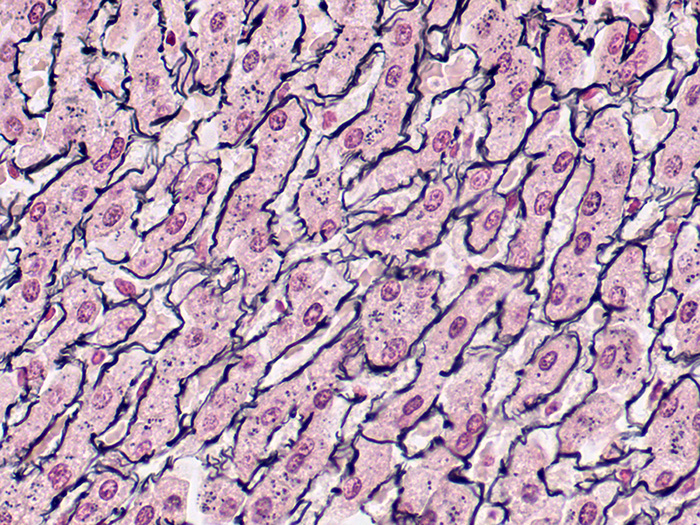
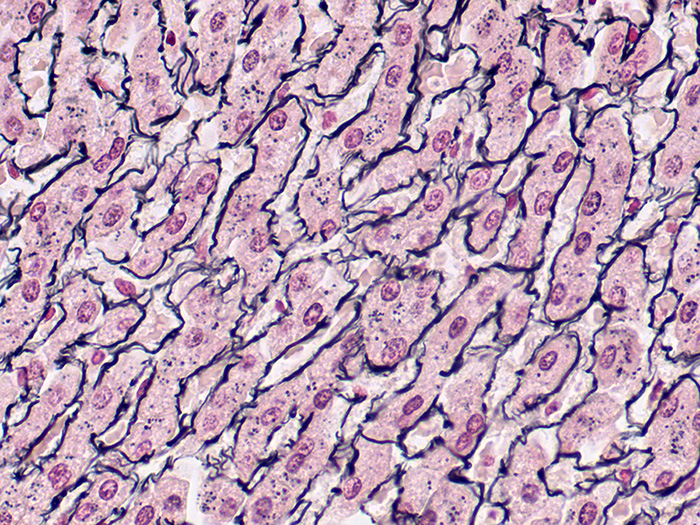
Reticulum
|
Validation Stain: Gordon-Sweets
Other Applicable Stains: Snook Reticulun and Gomori Stain for Reticular Fibers
|
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gordon & Sweets Reticulum quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Reticulum, Gordon & Sweets Stain Kit: | Part 9168A | Individual Stain Solution | |
| Solution A: | Potassium Permanganate 1%, Aqueous | 250 ml | Part 13393 |
| Solution B: | Oxalic Acid 1%, Aqueous | 250 ml | |
| Solution C: | Ferric Ammonium Sulfate 2.5%, Aqueous | 250 ml | |
| Solution D: | Silver Nitrate 10%, Aqueous | 50 ml | Part 13806 |
| Solution E: | Ammonium Hydroxide 28-30%, ACS | 50 ml | Part 1006 |
| Solution F: | Sodium Hydroxide 3%, Aqueous | 50 ml | |
| Solution G: | Formalin 10%, Aqueous | 250 ml | |
| Solution H: | Gold Chloride 0.2%, Aqueous | 250 ml | Part 11286 |
| Solution I: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution J: | Nuclear Fast Red Stain, Kernechtrot | 250 ml | Part 1255 |
APPLICATION:
Newcomer Supply Reticulum Control Slides are for the positive histochemical staining of reticulum fibers; regarded as specialized connective tissue fibers.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Ammoniacal Silver Working Solution. Save for Step #11.
-
- Place 5 ml of Solution D: Silver Nitrate 10%, Aqueous in a flask.
- Add Solution E: Ammonium Hydroxide 28-30%, ACS drop by drop, continuously swirling until formed precipitate completely dissolves. Do not add any excess Ammonium Hydroxide.
- Add 5 ml of Solution F: Sodium Hydroxide 3%, Aqueous.
- Re-dissolve formed precipitate with Solution E: Ammonium Hydroxide 28-30%, ACS until a faint cloudiness remains.
- If proceeded too far and no cloudiness remains, add Solution D: Silver Nitrate 10%, Aqueous drop by drop, until one drop causes solution to become permanently cloudy. Faint cloudiness is the optimum.
- Bring solution volume to 50 ml with distilled water; filter.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #3 and #4.
-
- Oxidize in Solution A: Potassium Permanganate 1%, Aqueous for 3 minutes.
- Wash in running tap water for 1 minute; rinse in distilled water.
- Bleach in Solution B: Oxalic Acid 1%, Aqueous for 2 minutes or until sections are colorless.
- Wash in running tap water for 1 minute; rinse in distilled water.
- Sensitize in Solution C: Ferric Ammonium Sulfate 2.5%, Aqueous; 10 to 15 minutes.
- Rinse in several changes of distilled water.
- Impregnate sections in filtered Ammoniacal Silver Working Solution (Step #3) for 2 minutes.
- Rinse well in running distilled water for 1 minute.
-
- See Procedure Note #5.
-
- Reduce in Solution G: Formalin 10%, Aqueous for 1 minute.
- Rinse in running tap water for 1 minute.
- Check control slide microscopically for sufficient black reticular fiber development.
-
- See Procedure Note #6.
-
- Tone in Solution H: Gold Chloride 0.2%, Aqueous for 1-2 minutes.
- Rinse well in distilled water.
- Place in Solution I: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash well in tap water for 1 minute; rinse in distilled water.
- Counterstain with Solution J: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
-
- Shake solution well before use; do not filter.
-
- Rinse well in distilled water.
-
- See Procedure Note #7.
-
- Quickly dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Reticular fibers | Black |
| Background | Red |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with silver solutions to prevent precipitation of silver salts. No metals of any kind should come in contact with silver solutions.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- This rinse step is critical for good reticulum demonstration. If rinsing is insufficient, excessive background staining may occur.
- If black reticular fibers are not evident or are lightly/poorly stained, return all slides to Ammoniacal Silver Working Solution (Step #11) and repeat Steps 11-14 with the same timings.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 177-179.
- Gordon, Harold, and Henry Sweets. “A Simple Method for the Silver Impregnation of Reticulum.” American Journal of Pathology4 (1936): 545-552.
- Modifications developed by Newcomer Supply Laboratory.