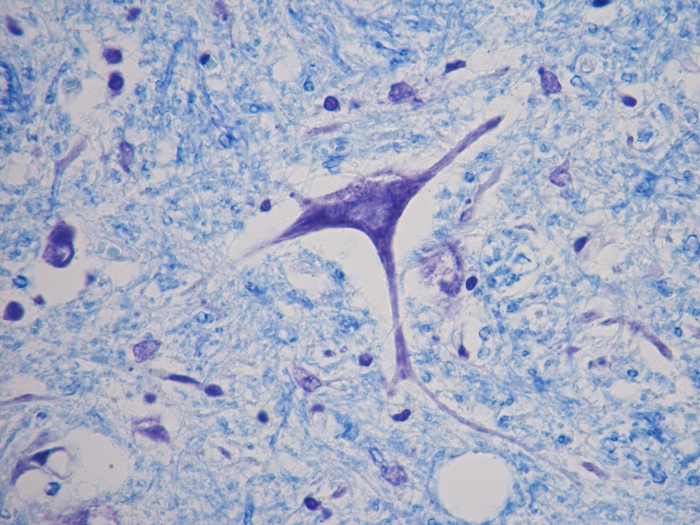
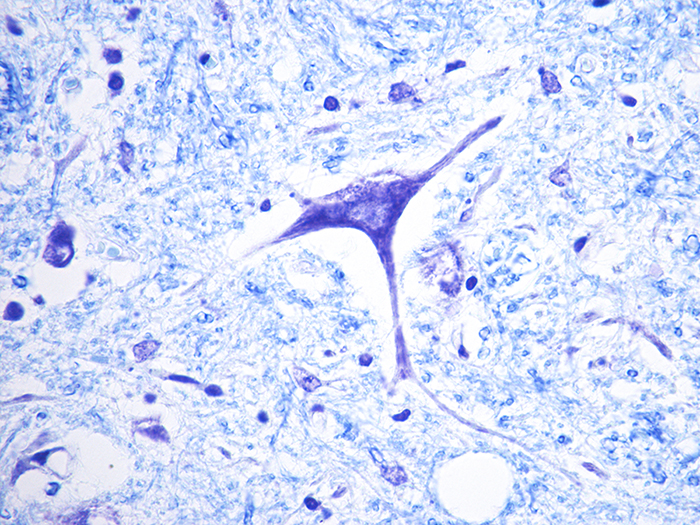
Luxol Fast Blue (LFB) - Cresyl Violet Stain Kit
![]()
Stains myelin and Nissl substance in central nervous system tissues and in peripheral nerve.
LUXOL FAST BLUE (LFB) – CRESYL VIOLET STAIN KIT INCLUDES:
| Part 9155A | ||
| Solution A: | Luxol Fast Blue Stain 0.1%, Alcoholic | 250 ml |
| Solution B: | Lithium Carbonate 0.5%, Aqueous | 250 ml |
| Solution C: | Cresyl Violet Stain, Aqueous | 250 ml |
| Solution D: | Acetic Acid 10%, Aqueous | 50 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Coplin Jar, Plastic | Part 5184 (for microwave modification) |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Luxol Fast Blue (LFB) – Cresyl Violet Stain Kit, with included microwave modification, is for the demonstration of myelin and Nissl substance in central nervous system and peripheral nerve tissues.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 8-10 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Prepare Working Lithium Carbonate 0.05%; combine and mix well;
-
- Solution B: Lithium Carbonate 0.5%, Aqueous 10 ml
- Distilled Water 90 ml
-
- Prepare Cresyl Violet Working Solution:
-
- Solution C: Cresyl Violet Stain, Aqueous 40 ml
- Solution D: Acetic Acid 10%, Aqueous 7 drops
- Combine, mix well and filter.
- Heat filtered solution in 57°C oven; hold for Step #13.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
-
- Stop at 95% ethyl alcohol; no distilled water rinse.
- See Procedure Notes #1 and #2.
-
- Incubate in Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic for 2 hours at 60°C or overnight at 37°C; cover tightly.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
Microwave Modification: See Procedure Note #3.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic. Microwave at 70°C for 10 minutes.
-
- Rinse quickly in 95% ethyl alcohol, 2-3 dips.
- Rinse in distilled water.
- Differentiate slides individually in Working Lithium Carbonate 0.05% (Step #2) for 10-15 seconds with agitation until gray matter and white matter are colorless and contrast with stained tissue.
- Further differentiate in 70% ethyl alcohol, until gray and white matter can be distinguished. Do not over differentiate.
- Rinse in distilled water.
- Check slides microscopically. Continue if additional differentiation is needed. Otherwise proceed directly to Step #13.
-
-
- One dip in Lithium Carbonate 0.05%, Aqueous (Step #2).
- Dip in two changes of 70% ethyl alcohol until green/blue white matter sharply contrasts with colorless gray matter.
-
- Rinse thoroughly in distilled water.
- Stain in heated Cresyl Violet Working Solution (Step #3) for 6 minutes in 57°C
- Rinse in distilled water.
- Dehydrate quickly to maintain Cresyl Violet Stain in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
RESULTS:
| Myelin | Blue |
| Nissl substance and nuclei | Violet |
| Neurons | Pink to violet |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 214-215.
- Klüver, Heinrich, and Elizabeth Barrera. “A Method for the Combined Staining of Cells and Fibers in the Nervous System.” Journal of Neuropathology and Experimental Neurology4 (1953): 400-403.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 494-495.
- Modifications developed by Newcomer Supply Laboratory.