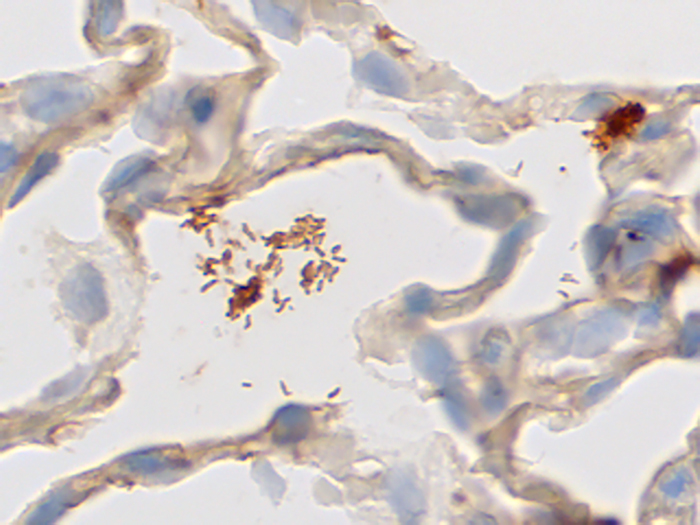
Helicobacter, Artificial
Organisms artificially introduced into a rat organ through a non-infective process.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung and negative staining human lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Helicobacter quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Helicobacter, Artificial Control Slides are for the positive immunohistochemical staining of spiral shaped Helicobacter bacterium, strongly associated with inflammation of the stomach and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
Helicobacter pylori purchased from American Type Culture Collection is used to produce the positive control tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Helicobacter Pylori primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Helicobacter positive expression | Golden to dark brown bacteria |
| Lung | Negative |
| Background staining possible | |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Helicobacter pylori Polyclonal is the primary antibody used along with Cell Marque detection and ancillary
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Cell Marque Helicobacter pylori Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.