BCL2
PRODUCT SPECIFICATIONS:
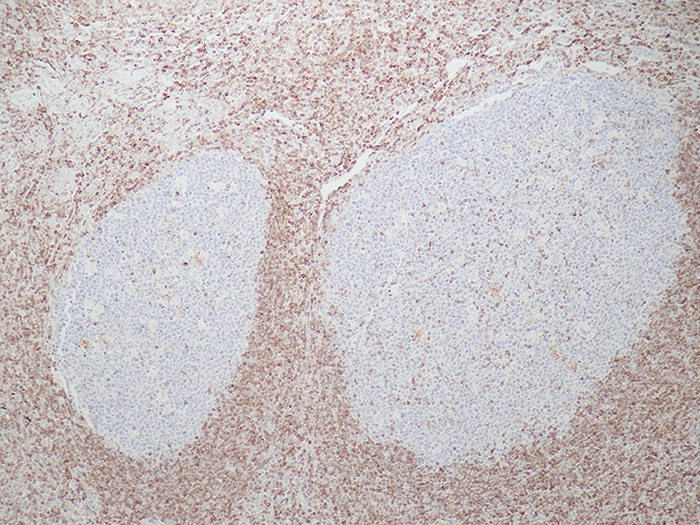
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: BCL2 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply BCL2 (B-cell lymphoma 2) Control Slides are for the positive immunohistochemical staining of BCL2. Frequently used to distinguish positive staining follicular lymphoma from negative reacting follicular hyperplasia.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply BCL2 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| BCL2 positive expression | Brown cytoplasmic staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque BCL2 (124) is the concentrated primary antibody used. Dilute primary antibody to 1/25 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque BCL2 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.