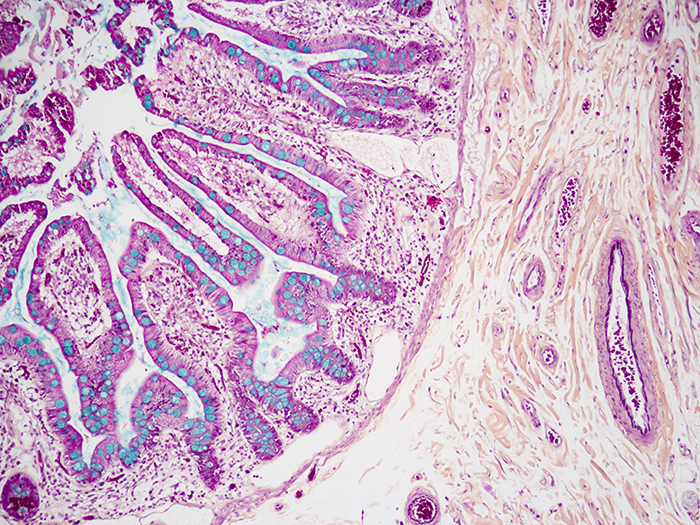
Movat-Russell Pentachrome
|
Validation Stain: Movat-Russell Modified Pentachrome
|
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine and positive staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Movat-Russell Pentachrome quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Movat-Russell Modified Pentachrome Stain Kit: | Part 9150A | Individual Stain Solution | |
| Solution A: | Alcian Blue Stain 1%, Aqueous | 250 ml | |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 50 ml | Part 1006 |
| Solution C: | Hematoxylin 10%, Alcoholic | 100 ml | |
| Solution D: | Ferric Chloride 10%, Aqueous | 100 ml | Part 10856 |
| Solution E: | Iodine, Verhoeff, Aqueous | 100 ml | Part 1209 |
| Solution F: | Ferric Chloride 2%, Aqueous | 250 ml | Part 108553 |
| Solution G: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution H: | Crocein Scarlet 7B Stain, Aqueous | 250 ml | |
| Solution I: | Acid Fuchsin Stain, Aqueous | 100 ml | |
| Solution J: | Phosphotungstic Acid 5%, Aqueous | 500 ml | Part 13345 |
| Solution K: | Orange G Stain 1%, Aqueous | 250 ml | |
| Solution L: | Acetic Acid 0.5%, Aqueous | 500 ml | Part 100121 |
APPLICATION:
Newcomer Supply Movat-Russell Pentachrome Control Slides are for the positive histochemical staining of connective tissue elements, mucin, fibrin, elastic fibers, muscle, and collagen.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Stain in Solution A: Alcian Blue Stain 1%, Aqueous for 20 minutes.
- Wash in running tap water for 5 minutes.
- Prepare fresh Alkaline Alcohol Solution; combine and mix well.
- Solution B: Ammonium Hydroxide 28-30% 5 ml
- Alcohol, Ethyl Denatured, 95% (Part 10842) 45 ml
- Place slides in fresh Alkaline Alcohol Solution for 30 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #3.
- Prepare fresh Hematoxylin Working Stain Solution just before use in the order given; combine and mix well.
- Solution C: Hematoxylin 10%, Alcoholic 10 ml
- Alcohol, Ethyl Denatured 100% (Part 10841) 10 ml
- Solution D: Ferric Chloride 10%, Aqueous 10 ml
- Solution E: Iodine, Verhoeff, Aqueous 10 ml
- Stain in fresh Hematoxylin Working Stain Solution for 15 minutes.
- Discard after successful differentiation in Step #11.
- Rinse in several changes of distilled water.
- Differentiate one slide at a time in Solution F: Ferric Chloride 2%, Aqueous until elastic fibers contrast sharply with the background; approximately 5-10 dips.
- See Procedure Note #4.
- Rinse in distilled water.
- Place in Solution G: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Prepare Crocein Scarlet-Acid Fuchsin Solution:
- Solution H: Crocein Scarlet 7B Stain, Aqueous 40 ml
- Solution I: Acid Fuchsin Stain, Aqueous 10 ml
- Stain in Crocein Scarlet-Acid Fuchsin Solution for 1 minute.
- Rinse in several changes of distilled water.
- Rinse in Solution L: Acetic Acid 0.5%, Aqueous for 30 seconds.
- Place in Solution J: Phosphotungstic Acid 5%, Aqueous; two changes of 5 minutes each.
- Rinse in Solution L: Acetic Acid 0.5%, Aqueous.
- Stain in Solution K: Orange G Stain 1%, Aqueous for 15 minutes.
- Dehydrate through three changes of 100% ethyl alcohol, 10 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Do not use 95% ethyl alcohol in the dehydration step.
RESULTS:
| Nuclei and elastic fibers | Black |
| Collagen and reticular fibers | Yellow |
| Ground substance and mucin | Blue |
| Fibrinoid, fibrin | Intense red |
| Muscle | Red |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- It is important to completely remove Alkaline Alcohol Solution with running tap water. Failure to do so will inhibit the subsequent staining steps.
- Do not over-differentiate in Solution F: Ferric Chloride 2%, Aqueous. If the background is completely colorless, the section may be over-differentiated. Over-differentiated sections may be restained in Hematoxylin Working Stain Solution (Step #9) provided sections have not been treated with an alcohol/dehydration step.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 172-174.
- Movat, Henry, “Demonstration of All Connective Tissue Elements in a Single Section”. AMA Archives of Pathology. 1955; 60 (3): 289–295.
- Russell H. K. Jr. “A Modification of Movat’s Pentachrome Stain”. AMA Archives of Pathology.1972; 94 (2): 187–191.
- Modifications developed by Newcomer Supply Laboratory.