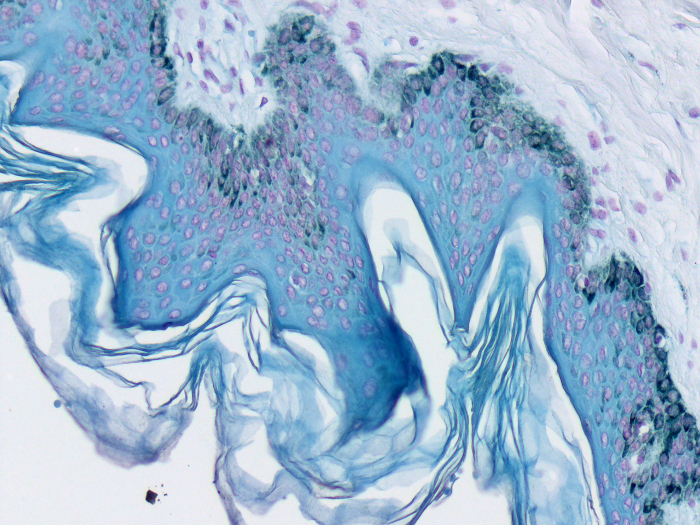
Schmorl Melanin Stain
Reagents for this procedure are sold as individual stain solutions and are available for purchase under separate part numbers with storage requirements and expiration date designated per bottle.
SOLUTIONS:
| 250 ml | 500 ml | |
| Ferric Chloride 1%, Aqueous | Part 10855A | Part 10855B |
| Potassium Ferricyanide 1%, Aqueous | Part 13390A | Part 13390B |
Additionally Needed:
| Melanin Control Slides | Part 4430 |
| Nuclear Fast Red Stain, Kernechtrot | Part 1255 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Schmorl Melanin Stain demonstrates sites of reduction activity in tissue sections. A positive reaction indicates the presence of melanin and other reducing substances such as; argentaffin, chromaffin, bile and formalin pigment.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
-
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Prepare Ferric Chloride-Potassium Ferricyanide Working Solution; combine and mix well.
-
- Ferric Chloride 1%, Aqueous 30 ml
- Potassium Ferricyanide 1%, Aqueous 10 ml
-
- Place in Ferric Chloride-Potassium Ferricyanide Working Solution for 5 to 10 minutes.
-
- See Procedure Note #3.
-
- Wash well in running tap water.
- Counterstain in Nuclear Fast Red Stain, Kernechtrot (Part 1255) for 5 minutes.
-
- Shake solution well before use; do not filter.
-
- Rinse well in distilled water.
-
- See Procedure Note #4.
-
- Dehydrate quickly through two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Melanin & other reducing substances | Blue |
| Nuclei | Pink-red |
| Cytoplasm | Pale pink |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Melanin will react quicker than other reducing substances; adjust reaction time accordingly.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 243-244.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 259-260.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 223.
- Modifications developed by Newcomer Supply Laboratory.