PRODUCT SPECIFICATIONS:
Cell Line: Two positive staining cores and one negative staining core from HistoCyte Cell Microarray HCL006
Fixation: Formalin 10%, Phosphate Buffered.
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Storage: 2-8°C in a light deprived and humidity controlled environment.
Expiration: Refer to individual product label.
Intended Use: Research Use Only (RUO).
PRODUCT DESCRIPTION:
The enclosed cell line control slides are developed from a process that allows production of compact cell preparations, cultured from human cell lines, that retain their cellular morphology and are tissue-like in composition. These cell lines are standardized and manufactured to provide consistent results from slide to slide. Each slide contains three, 1.5-2mm diameter cell line cores which demonstrate positive and negative expressions for the specific marker.
-
- A = Breast adenocarcinoma (negative p16 expression)
- B = Cervical adenocarcinoma (positive p16 expression)
- C = Epidermoid carcinoma (positive p16 expression)
APPLICATION:
Newcomer Supply p16 Cell Line Control Slides are appropriate for use in immunohistochemistry (IHC) and in situ hybridization (ISH). The slides may be used to check for reagent performance and troubleshooting of HPV ISH and p16 IHC staining. p16 can serve as a surrogate marker for high-risk HPV in cases of cervical, head and neck and a variety of HPV related carcinomas. It is the responsibility of the end user to determine suitability with their reagents and procedures within their laboratory.
These Histosette II tissue processing & embedding cassettes are suitable for hoppers accepting plastic sleeves such as Thermo Fisher printers. They load in cassette labeling instruments in one simple operation! Molded from acetal, these cassettes keep specimens safely submerged in liquid and are resistant to the chemical action of most histological solvents. The efficient flow-through slots maximize fluid exchange and ensure proper drainage.
PACKAGING OF THE HISTOSETTE II TISSUE PROCESSING & EMBEDDING CASSETTES IN QUICKLOAD SLEEVES:
- 75 cassettes/sleeve; 10 sleeves/case; 750 cassettes/case and 750 covers.
HISTOSETTE II TISSUE PROCESSING & EMBEDDING CASSETTES IN QUICKLOAD SLEEVES:
- Convenient plastic dispensing sleeve compatible with Thermo Fisher printers.
- Efficient flow-through slots maximize fluid exchange and ensure proper drainage.
- Lids are packaged separately and have a secure locking device which safely holds the specimen during processing.
- Anterior writing area is at a 45° angle.
- Cassettes also available with QuickLoad Taped Stacks (Part 5131).
These Histosette II Biopsy tissue processing & embedding cassettes are suitable for hoppers accepting plastic sleeves such as Thermo Fisher printers. They load in cassette labeling instruments in one simple operation! Molded from acetal, these cassettes keep specimens safely submerged in liquid and are resistant to the chemical action of most histological solvents. The efficient flow-through slots maximize fluid exchange and ensure proper drainage.
PACKAGING OF THE HISTOSETTE II BIOPSY TISSUE PROCESSING & EMBEDDING CASSETTES IN QUICKLOAD SLEEVES:
- 75 biopsy cassettes/sleeve; 10 sleeves/case; 750 biopsy cassettes/case and 750 covers.
HISTOSETTE II BIOPSY TISSUE PROCESSING & EMBEDDING CASSETTES IN QUICKLOAD SLEEVES:
- Convenient plastic dispensing sleeve compatible with Thermo Fisher printers.
- 1 mm square openings to maximize fluid exchange.
- Lids are packaged separately and have a secure locking device that safely holds the specimen during processing.
- Anterior writing area is at a 45° angle.
- Cassettes also available with QuickLoad Taped Stacks (Part 5132).
Enhance your histology experience with this advanced tissue flotation water bath, meticulously crafted for seamless operation. Digitally controlled for unparalleled ease, it boasts a deep water basin engineered for effortless handling of paraffin tissue sections.
Navigate effortlessly through its functionalities via the expansive color touch screen, offering intuitive controls for user settings. Illuminating your workflow, the glass basin is thoughtfully lit, ensuring optimal visibility for precise tissue placement.
Also, there is no external temperature probe that you will need to work around in the water basin. The bath’s design has the temperature sensor built into the device!
FEATURES OF THE TISSUE FLOTATION BATH – DIGITAL: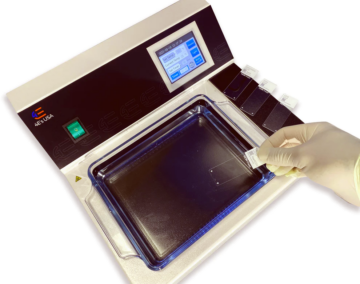
-
- Extra deep basin helps lift sections out of the bath easily
- Glass basin is lighted for easy tissue placement
- Large slide placement area
- Quiet fan-less design
- High Definition touch screen
- Temperature accuracy within 1°C
- Nonvolatile memory retains the last temperature set point after turning off
- Timer and alarm reminder
- Auto turn-on/turn-off function
SPECIFICATIONS:
-
- Temperature Range: 0°C-70°C
- Voltage: AC120V ±10%, 60Hz±1Hz
- Power: 200 Watts
- Dimensions: 12 ¾”L x 13 ¼”W x 6 ½”H
- Glass Basin Dimensions: 8 ¼”L x 6 ½”W x 2 ¼”H
- Relative Humidity: < 80%
- Temperature Accuracy: ±1°C
- Heating Time: 20 minutes to heat up to 54°C
- Exterior Material: Epoxy Painted Cold Rolled Steel
- Large slide placement area: Accommodates 8 slides
WARRANTY DETAILS:
-
- Two year warranty
Manual for Tissue Flotation Bath TFB200101
HistoCyte Laboratories’ NTRK Analyte Control contains two cell lines that demonstrate positive and negative expression of NTRK. It specifically expresses wild type (WT) TrkA, that is recognized by the pan-NTRK antibodies as the WT Trk proteins and fusion proteins share a highly homologous c-terminus, within which the tyrosine kinase domain resides. Ideal for use as a same slide quality control in immunohistochemistry (IHC) to demonstrate the reagents have been applied to the slide. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Large cell lymphoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
- Not suitable for FISH as there is no translocation in the cell line. It is WT NTRK1.
HistoCyte Laboratories’ MSH6 Analyte Control contains 2 cell lines, one with intact expression for MSH6 and one with loss of expression for MSH6. This two core product is a cost effective means of controlling for MSH6 assays over the four core MMR Analyte Control. However, it cannot be used with other MMR assays. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Colon adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ MSH2 Analyte Control contains 2 cell lines, one with intact expression for MSH2 and one with loss of expression for MSH2. This two core product is a cost effective means of controlling for MSH2 assays over the four core MMR Analyte Control. However, it cannot be used with other MMR assays. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Colon adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ MMR Analyte Control consists of four different cell lines with loss of and intact expression for MLH1, PMS2, MSH2 and MSH6. This product is a convenient quality control solution being suitable for use with all the MMR assays, regardless of which assay is used. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Prostate carcinoma
Cell line C: Colon adenocarcinoma
Cell line D: Colon adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ MLH1/PMS2 Analyte Control contains 2 cell lines, one with intact expression for MLH1 and PMS2 and one with loss of expression for MLH1 and PMS2. This two core product is a cost effective means of controlling for MLH1 and PMS2 assays over the four core MMR Analyte Control. However, it cannot be used with the other MMR assays. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Prostate carcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ HPV/p16 Analyte Control contains 3 cell lines that demonstrate high, medium and negative expression of high-risk human papillomavirus types 16 and 18 by in situ hybridization (ISH). The same cell lines also demonstrate high, intermediate (heterogeneous) and negative expression of p16 by immunohistochemistry (IHC). Ideal for use as a same slide control for HPV ISH and p16 IHC to demonstrate assay efficacy. These cell lines are derived from the following tumors:
Cell line A: Human breast adenocarcinoma
Cell line B: Human cervical adenocarcinoma
Cell line C: Human epidermoid carcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ multi-purpose Breast Analyte Control is a qualitative control that contains two cell lines that demonstrate positive and negative expression of ER, PR and HER2. Ideal for use as a same slide control in immunohistochemistry (IHC) to demonstrate the reagents have been correctly applied to the slide. These cell lines are derived from the following tumors:
Cell line A: Osteosarcoma
Cell line B: Breast ductal carcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ BRAF V600E Analyte Control consists of two cores negative and positive for expression of BRAF V600E. This provides a cost effective means on controlling for BRAF V600E in immunohistochemistry. These cell lines are derived from the following tumors:
Cell line A: Breast ductal carcinoma
Cell line B: Malignant melanoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ ALK-Lymphoma Analyte Control contains two cell lines that demonstrate positive and negative expression of NPM-ALK associated lymphoma. This is a cost effective same slide quality control for use in immunohistochemistry (IHC) to demonstrate the reagents have been applied to the slide. These cell lines are derived from the following tumors:
Cell line A: Breast Adenocarcinoma
Cell line B: Anaplastic large cell lymphoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ ROS1 Analyte ControlDR is the dynamic range version of the ROS1 control, containing an additional cell line with very low expression of ROS1. These cell lines are derived from the following tumors:
Cell line A: Breast Adenocarcinoma
Cell line B: Glioblastoma
Cell line C: Lung adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ PR Dynamic Range Analyte Control contains four cores, offering a range of expression for PR: Negative, Low/Intermediate, Intermediate/High, and High. These cell lines are derived from the following tumors:
Cell line A: Ductal Carcinoma
Cell line B: Breast Adenocarcinoma
Cell line C: Ductal Carcinoma
Cell line D: Ductal Carcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ PD-L1 Analyte ControlDR consists of four different cell lines with PD-L1 expression levels of high, intermediate, low and negative. Ideal for use as a same slide control for PD-L1 to demonstrate the sensitivity of your assay and improve confidence in the result. These cell lines are derived from the following tumors:
Cell line A: Breast ductal carcinoma
Cell line B: Osteosarcoma
Cell line C: Fibrosarcoma
Cell line D: T-cell non-Hodgkin lymphoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ HPV/p16 Analyte ControlDR contains 4 cell lines that demonstrate a full Dynamic Range of expression for high risk human papillomavirus types 16 and 18: high, medium, low and negative gene status by ISH. The same cell lines demonstrate homogenous, heterogenous and negative expression of p16. Ideal for use as same slide control for HPV in situ hybridization (ISH) for strains 16 and 18 and in p16 immunohistochemistry (IHC). These cell lines are derived from the following tumors:
Cell line A: Human breast adenocarcinoma
Cell line B: Human cervical squamous cell carcinoma
Cell line C: Human cervical adenocarcinoma
Cell line D: Human epidermoid carcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ ER Dynamic Range Analyte Control contains four cores, offering a full range of expression for ER: Negative, Low, Intermediate, and High. These cell lines are derived from the following tumors:
Cell line A: Osteosarcoma
Cell line B: Urothelial carcinoma
Cell line C: Non-small cell lung carcinoma
Cell line D: Breast adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ multipurpose Breast Analyte ControlDR contains five cell lines that demonstrate a Dynamic Range of 0, 1+ and 3+ for HER2. For ER and PR a variety of staining provides a degree of sensitivity over a standard control. Ideal for use in a laboratory where one control can be used across multiple assays as a same slide control in immunohistochemistry (IHC). These cell lines are derived from the following tumors:
Cell line A: Osteosarcoma
Cell line B: Breast ductal carcinoma
Cell line C: Breast ductal carcinoma
Cell line D: Breast ductal carcinoma
Cell line E: Breast ductal carcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ ALK-Lung Analyte Control is a qualitative control containing two cell lines that demonstrate positive and negative expression of EML4-ALK. This fusion protein is commonly associated with lung cancer. Ideal for use as a same slide control in immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) to demonstrate the reagents have been correctly applied to the slide. These cell lines are derived from the following tumors:
Cell line A: Breast Adenocarcinoma
Cell line B: Lung Adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ ALK Analyte ControlDR consists of four different cell lines expressing WT and ALK fusion proteins. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Glioblastoma
Cell line C: Lung adenocarcinoma
Cell line D: Anaplastic large cell lymphoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
The Slide Filing System Metal Cabinets are used in histology laboratories and hospitals and are designed for long term storage of large quantities of microscope slides.
SPECIFICATIONS OF THE SLIDE FILING SYSTEM METAL CABINETS:
-
- Six drawers holding up to 5,000 standard microscope slides
- Interlocking system for safe stacking
- Stack up to 12 units High
- Durable cold rolled steel construction with epoxy paint
- Each drawer comes with two foam blocks to maintain slides upright when drawers are partially filled
- Base available to raise bottom cabinet
DIMENSIONS OF THE SLIDE FILING SYSTEM METAL CABINETS AND BASE:
-
- Cabinet Dimensions: 18 ¾”L x 15 ¾”W x 5″H
- Base Dimensions: 18 ¾”L x 15 ¾”W x 5″H
CaviWipes 2.0 is a multi-purpose disinfectant for use in cleaning and disinfecting hard, non-porous, inanimate surfaces and non-critical instruments in hospitals, laboratories and other critical care areas where environmental control of cross-contamination between treated surfaces is important.
CAVIWIPES 2.0 SURFACE DISINFECTION TOWELETTES FEATURES & BENEFITS:
- 2-minute universal contact time for bacteria, viruses, and pathogenic fungi (Candida auris)
- Proven effective against SARS-CoV-2 on hard non-porous surfaces
- 1 step disinfecting (if no visible debris present on surface)
- Effective against 60 relevant pathogens including Norovirus and Candida auris for added confidence in preventing healthcare associated infections.
- Excellent Material Compatibility – Compatible with most non-porous, hard surfaces commonly used in clinical settings
QUALIFICATIONS:
- EPA’s Emerging Viral Pathogens claim for all virus types (enveloped, large and small non-enveloped viruses) to meet current and potentially future infection prevention needs
- On EPA List Q
CAVIWIPES 2.0 SURFACE DISINFECTION TOWELETTES LIST OF USES:
- Ambulance equipment surfaces
- Animal care facilities
- Bathrooms
- Correctional facilities
- Daycare centers
- Dental offices
- Emergency medical settings
- Emergency vehicles
- Exterior surfaces of anesthesia machines and respiratory therapy equipment
- Health club facilities
- Hospitals
- Infant/child care equipment surfaces
- Interior and exterior surfaces of infant incubators, bassinets
- Isolation areas
- Laboratories
- Laundry rooms
- Neonatal units
- Nursing homes
- Operating rooms
- Ophthalmic and optometric facilities
- Outpatient surgical centers
- Oxygen hoods
- Schools
- Surgical centers
CAVIWIPES 2.0 SURFACE DISINFECTION TOWELETTES KILL CLAIMS:
2 Minute Efficacy Against
- Adenovirus Type 2
- Echovirus Type 11
- Enterovirus Type 71
- Human Rotavirus
- Norovirus
- Adenovirus Type 5
- Rhinovirus Type 1A
Pathogenic Fungi
- Candida auris
Multi-Drug-Resistant Bacteria
- Penicillin-Resistant Streptococcus pneumoniae (PRSP)
- Vancomycin-Resistant Enterococcus faecalis (VRE)
- Methicillin-Resistant Staphylococcus aureus (MRSA)
- Carbapenem-resistant Klebsiella pneumoniae (CRKP)
- Multidrug-Resistant Acinetobacter baumannii (MRAB)
- NDM-1 Enterobacter cloacae
- Vancomycin-Intermediate Staphylococcus aureus (VISA)
- Methicillin-Resistant Staphylococcus epidermidis
- Multi-Drug Resistant Pseudomonas aeruginosa
- ESBL Klebsiella pneumoniae
- ESBL Escherichia coli
Bacteria
- Acinetobacter baumannii
- Bordetella pertussis
- Burkholderia cepacia
- Enterococcus faecium
- Escherichia coli O157:H7
- Klebsiella pneumoniae
- Legionella pneumophila
- Micrococcus luteus
- Moraxella catarrhalis
- Pseudomonas aeruginosa
- Salmonella enterica
- Staphylococcus aureus
- Stenotrophomonas maltophilia
- Streptococcus mutans
- Streptococcus pyogenes
- Klebsiella aerogenes
- Serratia marcescens
- Campylobacter jejuni
- Listeria monocytogenes
- Shigella dysenteriae
- Chlamydophila pneumoniae
- Flavobacterium columnare
- Mycobacterium tuberculosis (TB)
Enveloped Viruses
- Herpes simplex virus Type 1
- Herpes simplex virus Type 2
- Influenza A virus
- Varicella-zoster virus
- Cytomegalovirus
- SARS-associated Coronavirus
- SARS-CoV-2
- SARS-CoV-2-UK Variant
- SARS-CoV-2 South African Variant
- SARs-CoV-2-California Variant
- SARS-CoV-2 Delta Variant
- Influenza B virus
- Parainfluenza virus Type 3
- Human orthopneumovirus
- Measles virus
- Hepatitis C Virus (HCV)
- Hepatitis B Virus (HBV)
- Human Immunodeficiency Virus Type 1 (HIV-1)
SOLUTIONS:
| 250 ml | 500 ml | 1 Liter | 4 Liters | |
| Melanin Pigment Removal | ||||
| Potassium Permanganate 0.25%, Aqueous | Part 133931A | Part 133931B | ||
| Oxalic Acid 5%, Aqueous | Part 1293A | Part 1293B | ||
| Mercury Pigment Removal | ||||
| Melanin Control Slides | Part 4430 | |||
| Iodine, Gram, Aqueous OR Iodine, Lugol’s, Aqueous |
Part 1140A OR Part 12092A |
Part 1140C OR Part 12092B |
Part 1140E | |
| Sodium Thiosulfate 5%, Aqueous | Part 1389A | Part 1389B | ||
| Formalin Pigment Removal | ||||
| Picric Acid, Saturated Alcoholic | Part 1337A | Part 1337B |
For storage requirements and expiration date refer to individual product labels.
APPLICATIONS:
Newcomer Supply Pigment and Artifact Pigment Removal Technical Memo provides procedures for removal of pigments, both naturally occurring and artifact, from tissue sections.
Melanin pigment naturally occurs and is produced by melanocytes that provides skin, hair and eyes with color. When melanin pigment obscures cellular detail, it can be bleached with potassium permanganate and oxalic acid solutions.
Artifact pigments are produced in tissues during processing, often a result of fixation. Microscopically, these pigments usually appear to lie on top of the tissue and not within the cell.
- Mercury pigment is deposited after exposure to fixatives containing mercuric chloride. Sections must be treated for mercury pigment removal prior to staining.
- Formalin pigment results when acidic formalin solutions react with blood rich tissues such as spleen and areas of hemorrhage, forming brown or brownish-black crystalline birefringent substances. The use of Formalin 10%, Phosphate Buffered (Part 1090) assists in minimizing formalin pigment deposition.
METHOD:
Technique: Paraffin sections on adhesive slides
-
-
- See Procedure Note #1.
-
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
PROCEDURES:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- Melanin Pigment Removal:
-
- Two Melanin Control Slides (Part 4430) and two patient slides are needed.
- Label one control slide and one patient slide “with”.
- Label the other control slide and patient slide “without”. Set aside for Step h.
- Bleach “with” sections in Potassium Permanganate 0.25% Aqueous (Part 133931) for 5 to 20 minutes.
- Rinse in several changes of distilled water.
- Clear in Oxalic Acid 5%, Aqueous (Part 1293) for 1-2 minutes or until sections turn white.
- Wash in gently running tap water for 10 minutes.
- Stain as desired; including untreated melanin control and untreated patient slides labeled “without”.
- See Procedure Note #2.
-
-
- Mercury Pigment Removal:
-
- Treat sections with Iodine, Gram, Aqueous (Part 1140) or Iodine, Lugol’s, Aqueous (Part 12092) for 10 minutes.
- Wash briefly in running tap water.
- Place in Sodium Thiosulfate 5%, Aqueous (Part 1389) for 3 minutes.
- Wash in gently running tap water for 10 minutes.
- Stain as desired.
-
- Formalin Pigment Removal:
-
- Treat sections with Picric Acid, Saturated Alcoholic (Part 1337) for 10 minutes to 3 hours.
- Wash in gently running tap water for 10 minutes.
- Stain as desired.
- See Procedure Note #3.
-
- Mercury Pigment Removal:
PROCEDURE NOTES:
-
- Pigment removal procedures are harsh on tissues sections. The use of adhesive slides (Part 5070, 5079 or 6203) is recommended to ensure tissue adherence.
- The darker the melanin pigment the longer bleach will take to decolorize the pigment.
- Timing to remove formalin pigment will vary and will depend on the amount of pigment present in the sections.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 252-253.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 23-24, 254-255.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 130, 214, 220-221.
- Modifications developed by Newcomer Supply Laboratory.
HistoCyte Laboratories’ ROS1 Analyte Control consists of two cores negative and positive for expression of ROS1. This provides a cost effective means on controlling for ROS1 in immunohistochemistry and fluorescence in situ hybridization. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Lung adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
HistoCyte Laboratories’ specialized HER2 control has a full dynamic range of expression. Enhanced from multi-purpose breast analyte control to now include a 2+ cell line, this is specifically aimed at those wanting the most reliable, sensitive and accurate control for HER2. These cell lines are derived from the following tumors:
Cell line A: Breast adenocarcinoma
Cell line B: Breast adenocarcinoma
Cell line C: Gastric adenocarcinoma
Cell line D: Breast adenocarcinoma
- Cells are fixed in 10% neutral buffered formalin and paraffin wax embedded.
- Sections are cut at 4µm, mounted on positively charged slides and dried overnight at 37ºC.
- Cell microarrays (CMA) contain cores that are 1.5-2mm in diameter and 3-3.5mm in length. It is possible to obtain over 300 sections depending on thickness.
The Slide Staining Tray, 18 Place is ideal for clinical and research laboratory staining. It holds 18 standard microscope slides (76x26mm) securely in place for staining, rinsing and dry application.
FEATURES OF THE SLIDE STAINING TRAY, 18 PLACE:
-
- Durably constructed from solvent-resistant polypropylene blend
- Non-skid silicone feet keep tray in place
- Two ways to empty stain liquid: pour spouts on each corner or use drain hole
- Drain hole included with plug
- Equipped with handle cut-outs for easy handling
- Black lid included for light sensitive applications
- Humidity retention wells for specialized staining needs
- Proudly made in the USA
SPECIFICATIONS:
-
- Color: Black
- Capacity: 18 standard microscope slides (76 x 26 mm)
- Tray material: polypropylene with talc
- Feet & plug material: Silicone
- Operating temperature: 0 to 40°C
- Storage temperature: -20 to 60°C
- Tray Dimensions: 16.1 x 8.9 x 1.6 in (41 x 22.5 x 4 cm)
- Cover Dimensions: 15.7 x 8.4 x 0.4 in (39.8 x 21.4 x 1.1 cm)
INCLUDES:
-
- 1 Black Base/Tray
- 1 Black Cover
- 2 Drain Plugs
The Graduated Beakers are an excellent choice for mixing, pouring, stirring and measuring solutions in many laboratory operations.
SPECIFICATIONS OF THE GRADUATED BEAKERS:
-
-
- Made of translucent polypropylene for general laboratory work
- Bold, printed, dual scale graduations
- Single non-drip spout
- Chemically resistant to most acids, bases and many common solvents
- Autoclavable at 121°C
- Beakers without handles stack to save storage space
-
The Graduated Cylinders offer precise fluid measurements in many laboratory operations.
SPECIFICATIONS OF THE GRADUATED CYLINDERS:
-
- Made of translucent polypropylene for general laboratory work
- Easy to read molded graduations
- Wide openings with tapered pour spouts and a hexagonal base
- Chemically resistant to most acids, bases and many common solvents
- Working temperature range: 4° to 60°C
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Grocott Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, Grocott Methenamine Silver (GMS) Stain Kit: | Part 9121A/B | Individual Stain Solution | |
| Solution A: | Chromic Acid 5%, Aqueous | 250/500 ml | Part 10341 |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250/500 ml | Part 13821 |
| Solution C: | Silver Nitrate | 125/250 ml | Part 1142 |
| Solution D: | Methenamine Borate | 125/250 ml | Part 1142 |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250/500 ml | Part 11285 |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250/500 ml | Part 13888 |
| Solution G: | Light Green SF Yellowish Stain 0.02%, Aqueous | 250/500 ml | Part 12204 |
APPLICATION:
Newcomer Supply Fungus, GMS, Aspergillus, Animal Control Slides are for the positive histochemical staining of fungal organisms in tissue sections. The organism morphology is consistent with Aspergillus sp.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well:
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C – 60°C in a water bath 20-30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #6.
-
-
-
- Microwave Solution A: Chromic Acid 5%, Aqueous without slides in a plastic Coplin jar (Part 5184) for 1 minute at 60°C. Add slides to heated Solution A and oxidize for 10 minutes.
-
-
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash for 5 minutes in running tap water; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation.
-
Microwave Modification:
-
-
-
- Incubate slides in a plastic Coplin jar containing Silver-Methenamine Working Solution and microwave for 5 minutes at 45°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 3-5 minute intervals.
-
-
-
- Rinse in three to four changes of distilled water.
-
-
-
- Never use tap water at this step.
-
-
-
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 10-30 seconds.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish 0.02%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Fungus | Sharply outlined in black |
| Background | Green |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Maintain solution between 45°C-60°C to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th Chicago: ASCP Press, 2020. 221-226.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
SET INCLUDES:
| Part 1258A | ||
| Solution A: | Celestine Blue Stain 1%, Aqueous | 250ml |
| Solution B: | Ferric Ammonium Sulfate 4%, Aqueous | 250 ml |
Additionally Needed For H&E Staining:
| Hematoxylin and Eosin (H&E) Control Slides | Part 4278 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Acid Alcohol 1% | Part 10011 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Eosin Y Working Solution | Part 1072 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Newly Blue Stain Set provides a synthetic nuclear stain (hematoxylin substitute), that is indistinguishable from standard hematoxylin staining results. Newly Blue nuclear staining is crisp with well delineated purple to blue nuclei and displays a clear contrast to cytoplasmic stains for precise cellular interpretation.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Staining Sets are designed to be used with Coplin jar filled to 40 ml following the provided staining procedure.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Prepare Newly Blue Working Solution and mix well:
-
- Solution A: Celestine Blue Stain 1%, Aqueous 20 ml
- Solution B: Ferric Ammonium Sulfate 4%, Aqueous 20 ml
- Filter before use.
- See Procedure Notes #1 & #2.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #3 and #4.
-
- Stain with Newly Blue Working Solution for 4 minutes.
- Wash well in three changes of tap water.
- Differentiate quickly in Acid Alcohol 1% (Part 10011).
-
- Nuclei should be distinct; background light to colorless.
-
- Rinse well in three changes of tap water.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- Wash in three changes of tap water; rinse in distilled water.
- Drain excess water; proceed to 70% alcohol for 10 dips.
- Counterstain in Eosin Y Working Solution (Part 1072) or prepared Eosin-Phloxine Working Solution (Part 1082) for 30 seconds to 3 minutes, depending on preference of intensity.
- Dehydrate in two changes of 95% ethyl alcohol for 1 minute each and two changes of 100% ethyl alcohol, 10 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Nuclei | Blue |
| Cytoplasm and other tissue elements | Various shades of pink |
PROCEDURE NOTES:
-
- Newly Blue Working Solution is stable for up to 5 days.
- Blot off any surface sheen that may develop prior to use.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 115-116.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 148-150.
- Modifications developed by Newcomer Supply Laboratory.
The Hareta paraffin wax trimmer is an excellent choice for any histology lab that is searching for a quality, easy to use digitally controlled and affordable paraffin wax trimmer. It heats quickly to the user’s set temperature and will clean blocks fast and efficiently. Also comes with a wax collection tray that is easily removed and makes clean-up a breeze!
FEATURES OF THE PARAFFIN WAX TRIMMER-DIGITAL HARETA:
-
- Multiple blocks may be trimmed simultaneously
- Grooved surface for proper wax drainage into collection tray
- Digital Display
- Temperature range: Room Temp. to 99°C
- Precision of +/- 1°C
- Includes 5 disposable drip tray liners
DIMENSIONS & WEIGHT:
-
- Overall size: 9″ x 6″ x 7″
- Plate size: 9″ x 6″
- Weight: 5.5 lbs.
CERTIFICATION & APPROVALS:
-
- CE certified
| ELECTRICAL SPECIFICATIONS | ||||
|---|---|---|---|---|
| Voltage | Amps | Hertz | Wattage | Fuse Spec. |
| 110 | 0.5 | 60 | 120 | F1A/AC250V |
Histotechs will enjoy their work at a whole new level with this flotation bath where luxury and convenience merge!
This easy to use flotation bath offers a generous 8.5″ x 6.5″ x 2.5″(depth) glass basin that is nicely illuminated to float all of the ribbons one can cut. And with a built in slide warmer that can hold up to 20 slides, life doesn’t get much easier!
FEATURES OF THE DIGITAL HISTOLOGY WATER BATH:
-
- The glass basin is illuminated with adjustable LED lighting that has a super bright max intensity
- Separate On/Off timers for both the water bath and the slide warmer
- Magnetic bowl sensor contains no wires to break
- Water bath can be set from room temp. to 70°C
- 20 place slide warmer can be set from room temp. to 100ºC
- Will not overheat when dry
- Programmable to schedule ‘On’ and ‘Off’ times for each day of the week
DETAILS OF THE DIGITAL HISTOLOGY WATER BATH:
-
- Water Basin temp. range: room temp. to 70ºC
- Size Glass Basin (included): 210 x 170 x 55mm (depth)
- Glass Basin capacity: 52 fluid oz. (0.4 gallon)
- Drying Plate Size: 250 x 130mm (approximately 20 slides)
- Slide Warmer temp. range: room temp. to 100ºC
- Overall size: 310 x 460 x 135mm (height)
- Product weight: 7 kg
- Power requirements: 110-120 volt, 15A grounded outlet
CERTIFICATION AND APPROVALS:
-
- UL Approved
WARRANTY DETAILS:
-
- Two year warranty
- Three year on parts
Manual for Digital Tissue Flotation Bath KD-THII
Quick Start Guide for Digital Tissue Flotation Bath KD-THII
The GnomePen Flat Liquid Blocker has the same great GnomePen Classic Liquid Blocker features but generates a wide hydrophobic line to accommodate thick slices and Z-stack confocal microscopy. It forms a thin layer that does not interfere with cover slip application. It is optimized for tissue sections between 10-60 microns thick. Estimated coverage would be for approximately 250 slides.
SPECIFICATIONS OF GNOMEPEN FLAT:
-
-
- 7 ml volume: approximately 250 slides
- Fast drying time – 1 minute on the bench and under 30 seconds in chemical fume hood
- Shelf Life – 18 months
- Available in 6 colors: Blue, Red, Green, Orange, Purple & Yellow
- Clear body of pen
- Can be used on both silane coated and plain slides
- Insensitive to detergents & formaline/PFA fixatives
- Use with cryome and paraffin embedded slices
- Safe for antibodies and reagents
-
USE OF THE GNOMEPEN FLAT:
-
-
- Make sure glass surface is dry. If possible, apply before starting your assay.
- Shake the pen thoroughly (10-20 seconds) before use. If the pen was stored under 25°C, unscrew clockwise and re-screw before use.
- Clean the GnomePen tip. Use a kimwipe or soft paper wipe to clean the tip before starting.
- Hold the white tip of the pen and push the (clean) steel tip of the pen against the glass.
- Move quickly and draw a continuous line around your sample.
- Raise the pen off the surface once done.
- If a thicker line is needed or flow doesn’t begin instantly, gently squeeze the clear pen body.
- Allow pen line to fully dry before starting. Drying time is about 30 seconds in chemical fume hood (recommended) or 60 seconds on the bench.
-
SAFETY OF THE GNOMEPEN FLAT:
The main solvents in the GnomePen™ Flat are Toluene and Xylene. It is recommended that the pen be used in a chemical fume hood. Do not use GnomePen™ Flat near an open flame as both solvents are flammable.
STORAGE OF THE GNOMEPEN FLAT:
Store at room temperature away from open flame and direct sunlight. Cool, dark storage is recommended. Do not freeze the GnomePen Flat. Shelf life is 18 months.
If you are outside of North America, please visit the Invignome website for a distributor near you.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining organ
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 8 microns on Superfrost™ Plus slides.
Quality Control Stain: Bennhold Congo Red quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Amyloid, Bennhold Congo Red Stain Kit: | Part 9103A | Individual Stain Solution | |
| Solution A: | Congo Red Stain 1%, Aqueous | 250 ml | Part 1038 |
| Solution B: | Alkaline Alcohol | 250 ml | Part 1038 |
| Solution C: | Hematoxylin Stain, Mayer Modified | 250 ml | Part 1202 |
APPLICATION:
Newcomer Supply Amyloid Control Slides are for the positive histochemical staining of extraneous protein deposits in amyloidosis.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Place slides in Solution A: Congo Red Stain 1%, Aqueous for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #3.
-
-
-
- Place slides in a plastic Coplin jar (Part 5184) containing Solution A: Congo Red Stain 1%, Aqueous and microwave at 70°C for 3 minutes.
-
-
-
- Rinse in two to three changes of tap water; rinse in distilled water.
- Differentiate in Solution B: Alkaline Alcohol, 5 to 30 seconds, agitating constantly until slide background is cleared of Solution A: Congo Red Stain 1%, Aqueous.
- Rinse in two to three changes of tap water; rinse in distilled water.
- Counterstain with Solution C: Hematoxylin Stain, Mayer Modified, 3 to 5 minutes, depending on preference of nuclear stain intensity.
- Wash in running tap water for 5 to 10 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Light Field Microscopy: | ||
| Amyloid | Pink to red | |
| Nuclei | Blue | |
| Polarized Light: | ||
| Amyloid fluorescence | Apple green | |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- For optimal results cut sections at 8-10 microns to provide more intense staining and allow smaller amyloid deposits to be identified. Thinner sections may show faint staining and sections thicker than 8-10 microns may display yellow birefringence.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 366-367.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 177-178.
- Modifications developed by Newcomer Supply Laboratory.
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Fontana Masson quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
With Fontana Masson Stain Kit: Part 9105A Individual Stain Solution
Solution A: Silver Nitrate 10%, Aqueous 250 ml Part 13806
Solution B: Ammonium Hydroxide 28-30%, ACS 250 ml Part 1006
Solution C: Gold Chloride 0.2%, Aqueous 250 ml Part 11286
Solution D: Sodium Thiosulfate 5%, Aqueous 250 ml Part 1389
Solution E: Nuclear Fast Red Stain, Kernechtrot 250 ml Part 1255
APPLICATION:
Newcomer Supply Argentaffin Control Slides are for the positive histochemical staining of argentaffin substances in tissue sections.
PRESTAINING PREPARATION:
1. Heat dry sections in oven according to your laboratory protocol.
2. All glassware/plasticware must be acid cleaned prior to use.
a. See Procedure Notes #1 and #2.
3. Prepare Fontana Silver Working Solution (diamine silver) in an acid cleaned Erlenmeyer flask:
a. Solution A: Silver Nitrate 10%, Aqueous; 25 ml
b. Add Solution B: Ammonium Hydroxide 28-30%, ACS drop by drop, mix with swirling motion until solution clouds, then clears. Use caution to not add too much Solution B: Ammonium Hydroxide 28-30%, ACS.
c. Add more Solution A: Silver Nitrate 10%, Aqueous drop by drop until clear solution becomes slightly cloudy.
d. Let solution stand for 2-4 hours before use.
e. For use; after standing, filter the solution. Combine 20 ml of this filtered diamine silver solution with 40 ml of distilled water; 60 ml total.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
4. Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
a. See Procedure Notes #3 and #4..
5. Immerse slides in the Fontana Silver Working Solution (Step #3) in a 45°C to 60°C water bath for 1 hour.
6. Check slides microscopically; remove control, rinse in warm distilled water. Confirm that reaction is complete when granules are dark brown and background is colorless.
a. Return to heated Fontana Silver Working Solution for longer incubation if indicated.
7. Rinse well in three changes of distilled water.
8. Immerse in Solution C: Gold Chloride 0.2%, Aqueous; 10 minutes.
9. Rinse well in distilled water.
10. Place in Solution D: Sodium Thiosulfate 5%, Aqueous; 5 minutes.
11. Rinse well in distilled water.
12. Counterstain in Solution E: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
a. Shake solution well before use; do not filter.
13. Rinse well in distilled water.
a. See Procedure Note #5.
14. Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
CaviWipes™ Bleach disinfecting towelettes are non-woven disposable pre-saturated towelettes. The CaviWipes Bleach towelettes are effective against 43 relevant microorganisms.
CAVIWIPES BLEACH SURFACE DISINFECTION TOWELETTES FEATURES AND BENEFITS:
- Proven effective against SARS-CoV-2 on hard non-porous surfaces
- Kills all product labeled organisms in 3 minutes including C. diff, TB, drug resistant bacteria, viruses and fungi
- No PPE required*
- 1:10 Bleach dilution – Compatible with most medical device equipment**
- One-step cleaner and disinfectant*
- Low Odor
*Follow Special Cleaning and PPE instructions listed on Product label.
**See medical device equipment manual for compatibility information.
CAVIWIPES BLEACH SURFACE DISINFECTION TOWELETTES LIST OF USES:
- Ambulance equipment surfaces
- Animal care facilities
- Bathrooms
- Correctional facilities
- Daycare centers
- Dental offices
- Emergency medical settings
- Emergency vehicles
- Exterior surfaces of anesthesia machines and respiratory therapy equipment
- Health club facilities
- Hospitals
- Infant/child care equipment surfaces
- Interior and exterior surfaces of infant incubators, bassinets
- Isolation areas
- Laboratories
- Laundry rooms
- Neonatal units
- Nursing homes
- Operating rooms
- Ophthalmic and optometric facilities
- Outpatient surgical centers
- Oxygen hoods
- Schools
- Surgical centers
CAVIWIPES BLEACH SURFACE DISINFECTION TOWELETTES KILL CLAIMS:
3 Minute Efficacy Against:
Mycobacterium
- Mycobacterium tuberculosis var: bovis (BCG) (TB)
Non-Enveloped Viruses
- Adenovirus (type 2) (Adenoid 6)
- Canine Parvovirus
- Enterovirus (type D68) (EV-D68)
- Norovirus
- Hepatitis A (Virus) (Human HAV)
- Human Rotavirus (strain WA)
- Poliovirus (type 1), Chat Strain
- Rhinovirus (type 37)
Mold, Mildew, Fungi
- Aspergillus brasiliensis
- Candida albicans
- Candida auris
- Trichophyton interdigitale (formerly Trichophyton mentagrophytes)
Bacteria
- Burkholderia cepacia
- Clostridioides difficile spores
- Escherichia coli O157:H7
- Klebsiella pneumoniae
- Legionella pneumophila
- Listeria monocytogenes
- Neisseria gonorrhoeae
- Pseudomonas aeruginosa
- Salmonella enterica
- Serratia marcescens
- Staphylococcus aureus
- Streptococcus pyogenes
Drug-Resistant Bacteria
- Enterobacter cloacae
- Carbapenem-resistant Klebsiella pneumoniae (CRKP) (CRE)
- Extended spectrum-lactamase (ESBL) Escherichia coli
- Methicillin Resistant Staphylococcus aureus (MRSA)
- Multidrug Resistant (MDR) Acinetobacter baumannii
- Penicillin Resistant Streptococcus pneumoniae
- Vancomycin Resistant Enterococcus faecalis (VRE)
- Vancomycin Resistant Staphylococcus aureus (VRSA)
Enveloped Viruses
- Hepatitis C virus (HCV)
- Hepatitis B virus (HBV)
- Herpes simplex virus (type 1)
- Herpes simplex virus (type 2)
- HIV-1 (Human lmmunodeficiency Virus type 1)
- Human Coronavirus
- lnfluenza A virus Strain A (H3N2)
- lnfluenza B virus Strain B
- Measles virus
- Respiratory syncytial virus (RSV)
- SARS-CoV-2 (COVID-19 Virus)
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: AFB, Fite quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With AFB, Fite Stain Kit: | Part 91013A | Individual Stain Solution | |
| Solution A: | Xylene/Peanut Oil, 2:1 | 500 ml | Part 1449 |
| Solution B: | Carbol Fuchsin Stain, Ziehl-Neelsen | 250 ml | Part 1030 |
| Solution C: | Acid Alcohol 1% | 250 ml | Part 10011 |
| Solution D: | Light Green SF Yellowish 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
Newcomer Supply Fite, Leprosy, Animal Control Slides are for the positive histochemical staining of Mycobacterium leprae, the causative agent of leprosy.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen with high quality filter paper.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 10 minutes each.
-
- See Procedure Note #1.
-
- Drain slides, wipe off excess oil, and blot to opacity taking care to remove residual oil.
-
- See Procedure Note #2.
-
- Stain in freshly filtered Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature.
- Rinse well in distilled water.
- Differentiate slides individually in Solution C: Acid Alcohol 1% until sections are light pink; 5-10 dips.
- Rinse well in distilled water.
- Counterstain in Solution D: Light Green SF Yellowish 0.1%, Aqueous; 5-10 dips.
- Rinse in distilled water.
- Blot excess water from slide and air-dry or oven-dry completely.
- Dip dried slides in xylene and coverslip with a compatible mounting medium.
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 10 minutes each.
RESULTS:
| Mycobacterium leprae | Red |
| Other tissue elements | Green |
PROCEDURE NOTES:
-
- Acid-fastness of leprosy organisms is enhanced when the waxy capsule is protected by the mixture of xylene/peanut oil and avoidance of dehydrating solutions.
- It is important to blot well, residual oil may produce staining artifact.
- If using a xylene substitute, follow manufacturer’s recommendation for coverslipping step.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-instructional Text. 5th ed. Chicago: ASCP Press, 2020. 215-216.
- Fite, George, P.J. Cambre and M.H. Turner. “Procedure for Demonstrating Lepra Bacilli in Paraffin Sections”. Archives of Pathology 43 (1947). 624-625.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Brown-Brenn quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Gram, Brown-Brenn Stain Kit: | Part 9123A | Individual Stain Solution | |
| Solution A: | Crystal Violet-Oxalate Stain, Alcoholic | 250 ml | Part 10422 |
| Solution B: | Iodine, Gram, Aqueous | 250 ml | Part 1140 |
| Solution C: | Acetone-Alcohol 1:1 | 250 ml | Part 10016 |
| Solution D: | Basic Fuchsin Stain 0.25%, Aqueous | 250 ml | Part 1011 |
| Solution E: | Tartrazine Stain 0.25%. Acetic Aqueous | 250 ml | Part 14016 |
APPLICATION:
Newcomer Supply Gram Positive & Gram Negative Bacteria, Animal Control Slides are for the positive histochemical staining of gram positive and gram negative bacteria in a naturally occurring infection.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution A: Crystal Violet-Oxalate Stain, Alcoholic.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in freshly filtered Solution A: Crystal Violet-Oxalate Stain, Alcoholic (Step #2) for 1 minute.
- Rinse well in distilled water.
- Mordant in Solution B: Iodine, Gram, Aqueous for 1 minute.
- Rinse well in distilled water, removing excess iodine.
- Decolorize in Solution C: Acetone-Alcohol 1:1 until blue stops running; 7-10 dips.
- Rinse well in distilled water.
- Place in Solution D: Basic Fuchsin Stain 0.25%, Aqueous for 90 seconds.
- Rinse well in distilled water.
- Dip once in Solution C: Acetone-Alcohol 1:1.
- Counterstain in Solution E: Tartrazine Stain 0.25%, Acetic Aqueous for 5-15 seconds.
- Rinse well in distilled water.
- Dehydrate in two changes of 100% ethyl alcohol, 5 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
- Do not use 95% alcohol in the dehydration step.
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Gram positive bacteria | Blue/violet |
| Gram negative bacteria | Red |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 312-313.
- Brown, J.H., and L. Brenn. “A Method for the Differential Staining of Gram Positive and Gram Negative Bacteria in Tissue Sections”.Bulletin of The Johns Hopkins2 (1931): 69-73.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 188-189.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining submandibular gland and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: GCDFP-15 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply GCDFP-15 (gross cystic disease fluid protein 15) Control Slides are for the positive immunohistochemical staining of GCDFP-15, found in cyst fluid of cystic breast disease and a marker for primary and metastatic breast carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply GCDFP-15 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| GCDFP-15 positive expression | Brown cytoplasmic staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque GCDFP-15 (23A3) is the concentrated primary antibody used. Dilute primary antibody to 1/150 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque GCDFP-15 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PERIODIC ACID SCHIFF (PAS) STAIN KIT INCLUDES:
| Part 9162A | Part 9162B | ||
| Solution A: | Periodic Acid 0.5%, Aqueous | 250 ml | 500 ml |
| Solution B: | Schiff Reagent, McManus | 250 ml | 500 ml |
| Solution C: | Hematoxylin Stain, Harris | 250 ml | 500 ml |
| Solution D: | Acid Alcohol 1% | 250 ml | 500 ml |
| Solution E: | Lithium Carbonate, Saturated Aqueous | 250 ml | 500 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers at www.newcomersupply.com.
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Alpha Amylase 1%, Aqueous | Part 1905 (for glycogen digestion) |
| Coplin Jar, Plastic | Part 5184 (for glycogen digestion microwave modification) |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Periodic Acid Schiff (PAS) Stain Kit procedure, with methods for glycogen digestion, is used for staining glycoproteins and may aid in the differential diagnosis of tumors through detection of acid/neutral epithelial mucins and/or glycogen. Digestion steps can be employed for further identification of mucosubstances. PAS can also be used for staining basement membranes and fungal cell walls.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Proceed to Step #5 if not running Digestion.
- Digestion Step: See Procedure Note #3.
- Two control slides and two patient slides are needed.
- Label one control slide and one patient slide “with”.
- Label the other control slide and patient slide “without”.
- Place slides labeled “without” in separate Coplin jar of distilled water; hold for Step #5.
- Apply Alpha Amylase 1%, Aqueous (Part 1905) to slides labeled “with” for 30 minutes at room temperature.
- Proceed to Step #5.
- Digestion Microwave Modification: See Procedure Note #4.
- Follow Steps #3a through #3d.
- Place slides labeled “with” in a plastic Coplin jar containing Alpha Amylase 1%, Aqueous (Part 1905) and microwave for 1 minute at 37°C. Let sit in warm solution for an additional minute.
- Combine all slides for remaining steps; wash in running tap water for 1 minute, rinse in distilled water.
- Place in Solution A: Periodic Acid 0.5%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Solution B: Schiff Reagent, McManus for 20 minutes.
- Wash in lukewarm tap water for 5 minutes.
- Stain with Solution C: Hematoxylin Stain, Harris 1-5 minutes, depending on preference of nuclear stain intensity.
- Wash in tap water for 2-3 minutes.
- Differentiate in Solution D: Acid Alcohol 1%; 1-2 quick dips.
- Wash in tap water for 1 minute.
- Blue in Solution E: Lithium Carbonate, Saturated Aqueous; 3-4 dips.
- Wash in several changes of tap water; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Glycogen | Magenta |
| Glycogen digestion | Absence of magenta |
| Acid & neutral epithelial mucin | Magenta |
| Fungal cell walls | Red to purple |
| Basement membranes | Red to purple |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Slides labeled “with” will be treated with amylase digestion, slides labeled “without” will not be treated for digestion.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 168-171, 180.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.137-141.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 164-168, 245.
- Modifications developed by Newcomer Supply Laboratory.
Newcomer Supply PAP GnomePen Liquid Blocker creates a thin visible hydrophobic film around tissue sections to reduce the amount of reagent needed for tissue coverage and stain reaction. GnomePen has one of the thinnest PAP pen tips available, allowing for adjacent circles, lines or a drawn pattern to be applied on a single slide.
GnomePen contains a unique formulation that is water repellent, insoluble in alcohol and acetone, soluble in xylene and is insensitive to detergents (Triton X-100, Tween 20), varying pH and temperature. The GnomePen Classic PAP Pen is optimized for tissue sections between 4-20 microns thick. Estimated coverage would be for approximately 350 slides.
METHOD:
Technique: Paraffin, frozen sections and tissue culture cells
-
-
- Manual staining for:
- Immunohistochemistry (IHC) procedures
- Immunofluorescence Assay (IFA) procedures
- Manual staining for:
-
PROCEDURE:
-
-
- Shake pen thoroughly before use.
- Practice applying GnomePen liquid barrier on a test slide; push pen tip against the glass and apply a thin liquid barrier that dries to a film. If flow does not instantly begin, gently squeeze the pen body.
- Store GnomePen tightly capped; vertically with cap end up.
- Paraffin Section Method:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- Remove slide from water or buffer; blot excess solution from slide and tissue section. Or place long edges of slide on absorbent material to remove excess moisture.
- Encircle tissue section(s) on slide surface with GnomePen as illustrated. Do not touch the pen to any edge of the tissue.
- The liquid barrier can be drawn on a slightly damp slide.
- See Procedure Notes #1 and #2.
- Frozen Section and Tissue Culture Cells:
- Encircle frozen tissue section(s) or tissue culture cells on slide surface at room temperature with the GnomePen as illustrated. Do not touch the pen to any edges of the tissue or culture cells.
- See Procedure Notes #1 and #2.
- The liquid barrier should be applied before fixation or prior to the immersion of slide into water or buffer.
- After liquid barrier application, allow slide to dry in a flat position for 30-60 seconds at room temperature. Proceed with procedure when the drawn barrier has completely dried.
- See Procedure Note #3.
- Drain/rinse reagents off between staining steps; blotting slide and tissue as needed to remove excess solution.
- See Procedure Note #4.
- Complete staining and coverslip with a compatible mounting medium.
-
PROCEDURE NOTES:
-
-
- Once a tissue section is touched with GnomePen liquid barrier it cannot be removed. The section remains useable but slight colorization will result on the touched tissue.
- If the tissue section is not completely encircled or segregated by the GnomePen barrier film, reagents will not be fully retained on the tissue section and flood out onto the slide. This may compromise complete and adequate tissue coverage by reagents.
- If GnomePen barrier lines are not completely dry prior to staining, a precipitate from reaction with detection reagents may occur.
- The use of a Slide Moisture Chamber or StainTray (Part 68431, 6848 or 6847) is recommended for manual staining to maintain slide organization and a moist environment during the procedure.
-
REFERENCES:
-
-
- Grizzle, William, Cecil Stockard, and Paul Billings. “The Effects of Tissue Processing Variables Other Than Fixation on Histochemical Staining and Immunohistochemical Detection of Antigens.” The Journal of Histotechnology3 (2001): 213-219.
- Modifications developed by Newcomer Supply Laboratory.
-
If you are outside of North America, please visit the Invignome website for a distributor near you.
Retrieve easily and gently at 60°C for 30 minutes or 75°C for 15 minutes. Low temperature retrieval solution universally applicable to all antibodies and tissues. Also for IHC, Immunofluorescence, DNA & RNA probes. No Boiling. No High pH Buffer. No Citrate. Contains No Enzymes.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PAS quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Periodic Acid Schiff (PAS) Stain Kit: | Part 9162A/B | Individual Stain Solution |
| Solution A: Periodic Acid 0.5%, Aqueous | 250/500 ml | Part 13308 |
| Solution B: Schiff Reagent, McManus | 250/500 ml | Part 1371 |
| Solution C: Hematoxylin Stain, Harris | 250/500 ml | Part 12013 |
| Solution D: Acid Alcohol 1% | 250/500 ml | Part 10011 |
| Solution E: Lithium Carbonate, Saturated Aqueous | 250/500 ml | Part 12215 |
| Alpha Amylase 1%, Aqueous (for glycogen digestion) | Part 1905 | |
| Coplin Jar, Plastic (for glycogen digestion microwave modification) | Part 5184 | |
APPLICATION:
Newcomer Supply Periodic Acid Schiff (PAS) Glycogen Control Slides are for the positive histochemical staining of glycogen in tissue sections and can also be utilized as control slides for glycogen digestion steps.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Proceed to Step #5 if not running Digestion
- Digestion Step: See Procedure Note #3.
- Two control slides and two patient slides are needed.
- Label one control slide and one patient slide “with”.
- Label the other control slide and patient slide “without”.
- Place slides labeled “without” in separate Coplin jar of distilled water; hold for Step #5.
- Apply Alpha Amylase 1%, Aqueous (Part 1905) to slides labeled “with” for 30 minutes at room temperature.
- Proceed to Step #5.
- Microwave Modification: See Procedure Note #4.
- Follow Steps #3a through #3d.
- Place slides labeled “with” in a plastic Coplin jar containing Alpha Amylase 1%, Aqueous (Part 1905) and microwave for 1 minute at 37°C. Let sit in warm solution for an additional minute.
- Combine all slides for remaining steps; wash in running tap water for 1 minute; rinse in distilled water.
- Place in Solution A: Periodic Acid 0.5%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Solution B: Schiff Reagent, McManus for 20 minutes.
- Wash in lukewarm tap water for 5 minutes.
- Stain with Solution C: Hematoxylin Stain, Harris, 1 to 5 minutes, depending on preference of nuclear stain intensity.
- Wash in tap water for 2-3 minutes.
- Differentiate in Solution D: Acid Alcohol 1%; 1-2 quick dips.
- Wash in tap water for 1 minute.
- Blue in Solution E: Lithium Carbonate, Saturated Aqueous; 3-4 dips.
- Wash in several changes of tap water; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Glycogen digestion | Absence of magenta |
| Glycogen | Magenta |
| Acid & neutral epithelial mucin | Magenta |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Slides labeled “with” will be treated with amylase digestion, slides labeled “without” will not be treated for digestion.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 168-171, 180.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.137-141.
- Sheehan, Dezna C., and Barbara B.Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 164-168.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gordon & Sweets Reticulum quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Reticulum, Gordon & Sweets Stain Kit: | Part 9168A | Individual Stain Solution | |
| Solution A: | Potassium Permanganate 1%, Aqueous | 250 ml | Part 13393 |
| Solution B: | Oxalic Acid 1%, Aqueous | 250 ml | |
| Solution C: | Ferric Ammonium Sulfate 2.5%, Aqueous | 250 ml | |
| Solution D: | Silver Nitrate 10%, Aqueous | 50 ml | Part 13806 |
| Solution E: | Ammonium Hydroxide 28-30%, ACS | 50 ml | Part 1006 |
| Solution F: | Sodium Hydroxide 3%, Aqueous | 50 ml | |
| Solution G: | Formalin 10%, Aqueous | 250 ml | |
| Solution H: | Gold Chloride 0.2%, Aqueous | 250 ml | Part 11286 |
| Solution I: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution J: | Nuclear Fast Red Stain, Kernechtrot | 250 ml | Part 1255 |
APPLICATION:
Newcomer Supply Reticulum Control Slides are for the positive histochemical staining of reticulum fibers; regarded as specialized connective tissue fibers.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Ammoniacal Silver Working Solution. Save for Step #11.
-
- Place 5 ml of Solution D: Silver Nitrate 10%, Aqueous in a flask.
- Add Solution E: Ammonium Hydroxide 28-30%, ACS drop by drop, continuously swirling until formed precipitate completely dissolves. Do not add any excess Ammonium Hydroxide.
- Add 5 ml of Solution F: Sodium Hydroxide 3%, Aqueous.
- Re-dissolve formed precipitate with Solution E: Ammonium Hydroxide 28-30%, ACS until a faint cloudiness remains.
- If proceeded too far and no cloudiness remains, add Solution D: Silver Nitrate 10%, Aqueous drop by drop, until one drop causes solution to become permanently cloudy. Faint cloudiness is the optimum.
- Bring solution volume to 50 ml with distilled water; filter.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #3 and #4.
-
- Oxidize in Solution A: Potassium Permanganate 1%, Aqueous for 3 minutes.
- Wash in running tap water for 1 minute; rinse in distilled water.
- Bleach in Solution B: Oxalic Acid 1%, Aqueous for 2 minutes or until sections are colorless.
- Wash in running tap water for 1 minute; rinse in distilled water.
- Sensitize in Solution C: Ferric Ammonium Sulfate 2.5%, Aqueous; 10 to 15 minutes.
- Rinse in several changes of distilled water.
- Impregnate sections in filtered Ammoniacal Silver Working Solution (Step #3) for 2 minutes.
- Rinse well in running distilled water for 1 minute.
-
- See Procedure Note #5.
-
- Reduce in Solution G: Formalin 10%, Aqueous for 1 minute.
- Rinse in running tap water for 1 minute.
- Check control slide microscopically for sufficient black reticular fiber development.
-
- See Procedure Note #6.
-
- Tone in Solution H: Gold Chloride 0.2%, Aqueous for 1-2 minutes.
- Rinse well in distilled water.
- Place in Solution I: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash well in tap water for 1 minute; rinse in distilled water.
- Counterstain with Solution J: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
-
- Shake solution well before use; do not filter.
-
- Rinse well in distilled water.
-
- See Procedure Note #7.
-
- Quickly dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Reticular fibers | Black |
| Background | Red |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with silver solutions to prevent precipitation of silver salts. No metals of any kind should come in contact with silver solutions.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- This rinse step is critical for good reticulum demonstration. If rinsing is insufficient, excessive background staining may occur.
- If black reticular fibers are not evident or are lightly/poorly stained, return all slides to Ammoniacal Silver Working Solution (Step #11) and repeat Steps 11-14 with the same timings.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 177-179.
- Gordon, Harold, and Henry Sweets. “A Simple Method for the Silver Impregnation of Reticulum.” American Journal of Pathology4 (1936): 545-552.
- Modifications developed by Newcomer Supply Laboratory.
FUNGUS, GROCOTT METHENAMINE SILVER (GMS) STAIN KIT INCLUDES:
| Part 9121A | Part 9121B | ||
| Solution A: | Chromic Acid 5%, Aqueous | 250 ml | 500 ml |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250 ml | 500 ml |
| Solution C: | Silver Nitrate | 125 ml | 250 ml |
| Solution D: | Methenamine Borate | 125 ml | 250 ml |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250 ml | 500 ml |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250 ml | 500 ml |
| Solution G: | Light Green SF Yellowish Stain 0.2%, Aqueous | 250 ml | 500 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Hydrochloric Acid 5%, Aqueous | Part 12086 (for acid cleaning glassware) |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Coplin Jar, Plastic | Part 5184 (for microwave modification) |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Fungus, Grocott Methenamine Silver (GMS) Stain Kit procedure, with included microwave modification, is one of the best staining methods to demonstrate a variety of fungal organisms including: Pneumocystis, Aspergillus, Blastomyces, Candida and Histoplasma.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns.
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well.
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath approximately 20 to 30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
Microwave Modification: See Procedure Note #6
-
-
- Oxidize slides in a plastic Coplin jar containing Solution A: Chromic Acid 5%, Aqueous and microwave for 1 minute and 20 seconds at 60°C.
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash for 5 minutes in running tap water; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Pneumocystis may take longer to stain than other fungus.
- Staining at room temperature will require longer incubation.
-
- Microwave Modification:
-
- Incubate slides in a plastic Coplin jar containing Silver-Methenamine Working Solution and microwave for 1 minute at 70°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 2-3 minute intervals.
-
- Rinse in three to four changes of distilled water.
-
- Never use tap water at this step.
-
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 20 seconds to 1 minute.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.2%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Fungi | Crisp black cell walls with visible internal structures |
| Background | Green |
| Mucin | Taupe to dark gray |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Staining at higher temperatures means faster development but may cause precipitate to form in the working silver solution and deposit on slides. Maintaining silver solution between 45°C-60°C will minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 239-243.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 500 ml | |
| Alpha Amylase 1%, Aqueous | Part 1905B |
Additionally Needed:
| Periodic Acid Schiff (PAS) Glycogen Control Slides | Part 4540 |
| Periodic Acid 0.5%, Aqueous | Part 13308 |
| Schiff Reagent, McManus | Part 1371 |
| Hematoxylin Stain, Harris | Part 12013 |
| Acid Alcohol 1% | Part 10011 |
| Lithium Carbonate, Saturated Aqueous | Part 12215 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Coplin Jar, Plastic | Part 5184 (for glycogen digestion microwave modification) |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Alpha Amylase 1%, Aqueous is a convenient ready-to-use glycogen digestion solution for aiding in further identification of mucosubstances used in conjunction with the Periodic Acid Schiff (PAS) Stain procedure.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Digestion Step: See Procedure Note #3.
- Two control slides and two patient slides are needed.
- Label one control slide and one patient slide “with”.
- Label the other control slide and patient slide “without”.
- Place slides labeled “without” in separate Coplin jar of distilled water; hold for Step #5.
- Apply Alpha Amylase 1%, Aqueous to slides labeled “with” for 30 minutes at room temperature.
- Proceed to Step #5.
- Digestion Microwave Modification: See Procedure Note #4.
- Follow Steps #3a through #3d.
- Place slides labeled “with” in a plastic Coplin jar containing Alpha Amylase 1%, Aqueous and microwave for 1 minute at 37°C. Let sit in warm solution for an additional minute.
- Combine all slides for remaining steps; wash in running tap water for 1 minute, rinse in distilled water.
- Place in Periodic Acid 0.5%, Aqueous (Part 13308) for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Schiff Reagent, McManus (Part 1371) for 20 minutes.
- Wash in lukewarm tap water for 5 minutes.
- Stain with Hematoxylin Stain, Harris (Part 12013), 1-5 minutes, depending on preference of nuclear stain intensity.
- Wash in tap water for 2-3 minutes.
- Differentiate in Acid Alcohol 1% (Part 10011); 1-2 quick dips.
- Wash in tap water for 1 minute.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215); 3-4 dips.
- Wash in several changes of tap water; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Glycogen | Magenta |
| Glycogen digestion | Absence of magenta |
| Acid & neutral epithelial mucin | Magenta |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Slides labeled “with” will be treated with amylase digestion, slides labeled “without” will not be treated for digestion.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 168-171, 180.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.137-141.
- Sheehan, Dezna C., and Barbara B.Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 164-168.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo 1: Schiff Reagent, McManus for Periodic Acid Schiff (PAS) Stain
SOLUTION:
| 125 ml | 250 ml | 500 ml | 1 Liter | 4 Liters | |
| Schiff Reagent, McManus | Part 1371A | Part 1371E | Part 1371B | Part 1371C | Part 1371D |
Additionally Needed For Periodic Acid Schiff (PAS) Stain:
| Periodic Acid Schiff (PAS) Glycogen Control Slides | Part 4540 |
| Periodic Acid 0.5%, Aqueous | Part 13308 |
| Hematoxylin Stain, Harris Modified | Part 1201 |
| Acid Alcohol 1% | Part 10011 |
| Lithium Carbonate, Saturated Aqueous | Part 12215 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Schiff Reagent, McManus a crucial element in the Periodic Acid Schiff (PAS) Stain and for staining glycoproteins, epithelial mucins, basement membranes and fungal cell walls. Schiff Reagent, McManus is used in a variety of staining procedures that include:
-
- PAS with and without diastase
- Alcian Blue/PAS for mucins
- PAS/Light Green for Fungus
- Feulgen Reaction for demonstration of DNA
Schiff Reagent, McManus is a stable and reliable product that is conveniently stored at room temperature for ready-to-use staining. Although initially colorless, Schiff Reagent, McManus when in direct contact with skin, clothing, countertops, floors and other surfaces, will react and leave a bright magenta stain that is difficult to remove.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions manufactured by Newcomer Supply, Inc.
Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below.
PAS STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place in Periodic Acid 0.5%, Aqueous (Part 13308) for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Schiff Reagent, McManus for 20 minutes.
- See Procedure Notes #3, #4 and #5.
- Wash slides in lukewarm tap water for 5-10 minutes.
- Stain with Hematoxylin Stain, Harris Modified (Part 1201), 1-5 minutes, depending on preference of nuclear stain intensity.
- Wash in tap water for 2-3 minutes.
- Differentiate in Acid Alcohol 1% (Part 10011); 1-2 quick dips.
- Wash in tap water for 1 minute.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215); 3-4 dips.
- Wash in several changes of tap water; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Glycogen | Magenta |
| Acid & neutral epithelial mucin | Magenta |
| Fungal cell walls | Red to purple |
| Basement membranes | Red to purple |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Once used, do not pour Schiff Reagent, McManus back into the original bottle and/or mix with fresh solution.
- To test quality of Schiff Reagent, McManus reactivity;
- All glassware/plasticware containing Schiff Reagent, McManus should be thoroughly rinsed with running tap water to remove residual stain prior to standard glassware cleaning.
- Digestion steps can be employed in the PAS procedure for further identification of mucosubstances.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 168-174, 180.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.137-141.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 164-168, 245.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo 2: Schiff Reagent, McManus for Fungus, PAS/Light Green
SOLUTION:
| 125 ml | 250 ml | 500 ml | 1 Liter | 4 Liters | |
| Schiff Reagent, McManus | Part 1371A | Part 1371E | Part 1371B | Part 1371C | Part 1371D |
Additionally Needed For Fungus Stain, PAS/Light Green:
| Fungus, PAS, Aspergillus sp., Artificial Control Slides OR Fungus, PAS, Candida sp., Artificial Control Slides |
Part 4232 OR Part 4233 |
| Periodic Acid 0.5%, Aqueous | Part 13308 |
| Light Green SF Yellowish Stain 0.1%, Aqueous | Part 12203 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Schiff Reagent, McManus, a crucial element in the Fungus, PAS/Light Green procedure, is used for staining fungus and glycogen in tissue sections.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin section cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below.
FUNGUS, PAS/LIGHT GREEN STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place in Periodic Acid 0.5%, Aqueous (Part 13308) for 5 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Drain slides of excess water and stain in Schiff Reagent, McManus for 20 minutes.
- Wash gently in lukewarm tap water for 10 minutes to allow pink color to develop.
- Counterstain in Light Green SF Yellowish Stain 0.1%, Aqueous (Part 12203) for 5 seconds.
- See Procedure Note #3.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Fungal cell walls and glycogen | Red to magenta |
| Background | Pale green |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Increase or decrease staining time in Light Green SF Yellowish Stain 0.1%, Aqueous for preference of counterstain intensity.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 321-323.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245.
- Modifications developed by Newcomer Supply Laboratory.
LUXOL FAST BLUE (LFB) – CRESYL VIOLET STAIN KIT INCLUDES:
| Part 9155A | ||
| Solution A: | Luxol Fast Blue Stain 0.1%, Alcoholic | 250 ml |
| Solution B: | Lithium Carbonate 0.5%, Aqueous | 250 ml |
| Solution C: | Cresyl Violet Stain, Aqueous | 250 ml |
| Solution D: | Acetic Acid 10%, Aqueous | 50 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Coplin Jar, Plastic | Part 5184 (for microwave modification) |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Luxol Fast Blue (LFB) – Cresyl Violet Stain Kit, with included microwave modification, is for the demonstration of myelin and Nissl substance in central nervous system and peripheral nerve tissues.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 8-10 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Prepare Working Lithium Carbonate 0.05%; combine and mix well;
-
- Solution B: Lithium Carbonate 0.5%, Aqueous 10 ml
- Distilled Water 90 ml
-
- Prepare Cresyl Violet Working Solution:
-
- Solution C: Cresyl Violet Stain, Aqueous 40 ml
- Solution D: Acetic Acid 10%, Aqueous 7 drops
- Combine, mix well and filter.
- Heat filtered solution in 57°C oven; hold for Step #13.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
-
- Stop at 95% ethyl alcohol; no distilled water rinse.
- See Procedure Notes #1 and #2.
-
- Incubate in Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic for 2 hours at 60°C or overnight at 37°C; cover tightly.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
Microwave Modification: See Procedure Note #3.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic. Microwave at 70°C for 10 minutes.
-
- Rinse quickly in 95% ethyl alcohol, 2-3 dips.
- Rinse in distilled water.
- Differentiate slides individually in Working Lithium Carbonate 0.05% (Step #2) for 10-15 seconds with agitation until gray matter and white matter are colorless and contrast with stained tissue.
- Further differentiate in 70% ethyl alcohol, until gray and white matter can be distinguished. Do not over differentiate.
- Rinse in distilled water.
- Check slides microscopically. Continue if additional differentiation is needed. Otherwise proceed directly to Step #13.
-
-
- One dip in Lithium Carbonate 0.05%, Aqueous (Step #2).
- Dip in two changes of 70% ethyl alcohol until green/blue white matter sharply contrasts with colorless gray matter.
-
- Rinse thoroughly in distilled water.
- Stain in heated Cresyl Violet Working Solution (Step #3) for 6 minutes in 57°C
- Rinse in distilled water.
- Dehydrate quickly to maintain Cresyl Violet Stain in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
RESULTS:
| Myelin | Blue |
| Nissl substance and nuclei | Violet |
| Neurons | Pink to violet |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 214-215.
- Klüver, Heinrich, and Elizabeth Barrera. “A Method for the Combined Staining of Cells and Fibers in the Nervous System.” Journal of Neuropathology and Experimental Neurology4 (1953): 400-403.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 494-495.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Cell Line: One positive staining core and one negative staining core from HistoCyte Cell Microarray HCL012.
Fixation: Formalin 10%, Phosphate Buffered.
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Storage: 2-8°C in a light deprived and humidity controlled environment.
Expiration: Refer to individual product label.
Intended Use: Research Use Only (RUO).
PRODUCT DESCRIPTION:
The enclosed cell line control slides are developed from a process that allows production of compact cell preparations, cultured from human cell lines, that retain their cellular morphology and are tissue-like in composition. These cell lines are standardized and manufactured to provide consistent results from slide to slide. Each slide contains two, 1.5-2mm diameter cell line cores which demonstrate positive and negative expression for the specific marker.
A = Breast adenocarcinoma (negative ALK-Lymphoma expression)
B = Anaplastic large cell lymphoma (positive ALK-Lymphoma expression)
APPLICATION:
Newcomer Supply ALK-Lymphoma Cell Line Control Slides contain a cell line which has an NPM-ALK translocation and are suitable for use in immunohistochemistry (IHC) and in situ hybridization (ISH). The slides may be used to check for reagent performance and troubleshooting of ALK (anaplastic lymphoma kinase) staining. It is the responsibility of the end user to determine suitability with their reagents and procedures within their laboratory.
Convenient slide holder made of heavy duty plastic. Tray designed to hold slide in vertical position for easy left to right reading. Slides rest at a slightly upward angle. Designed for easy tipping of slide to pick up. Stackable trays so that slide will not touch tray above. Holds 20 slides (3″ x 1″). Dimensions: 8 1/8″ x 11 3/4″ x 11/16″ H). Available in 4 colors.
HELICOBACTER, TOLUIDINE BLUE/ALCIAN YELLOW STAIN KIT INCLUDES:
| Part 9130A | ||
| Solution A: | Periodic Acid 1%, Aqueous | 250 ml |
| Solution B: | Sodium Metabisulfite 5%, Acidified | 250 ml |
| Solution C: | Alcian Yellow Stain 1%, Alcoholic | 250 ml |
| Solution D: | Toluidine Blue Stock Stain 1%, Aqueous | 10 ml |
| Solution E: | Sodium Hydroxide 3%, Aqueous | 10 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Helicobacter, Toluidine Blue/Alcian Yellow Stain Kit procedure is used for screening and detection of Helicobacter pylori in gastrointestinal biopsy specimens.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
STAINING PROCEDURE:
-
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Oxidize in Solution A: Periodic Acid 1%, Aqueous; 10 minutes.
- Wash slides well with tap water.
- Place in Solution B: Sodium Metabisulfite 5%, Acidified; 5 minutes.
- Wash in tap water for 5 minutes.
- Stain in Solution C: Alcian Yellow Stain 1%, Alcoholic; 5 minutes.
- Wash well in tap water.
- Prepare fresh Toluidine Blue Working Solution; combine, mix well.
-
- Distilled Water 50 ml
- Solution D: Toluidine Blue Stock Stain 1%, Aqueous 5 ml
- Solution E: Sodium Hydroxide 3%, Aqueous 2 drops
-
- Stain in fresh Toluidine Blue Working Solution; 3 minutes.
- Wash well in tap water.
- Allow slides to air-dry completely.
- Clear dried slides in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
- Eliminate alcohol dehydration to retain organism staining.
-
RESULTS:
| Helicobacter pylori | Blue |
| Mucin | Yellow |
| Background | Pale blue |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 225-226.
- Leung, Jennifer, Kevin Gibbon and Robert Vartaniam. “Rapid Staining Method for Helicobacter pylori in Gastric Biopsies.” The Journal of Histotechnology 12 (1996): 131-132.
- Modifications developed by Newcomer Supply Laboratory.
ALCIAN BLUE/PAS STAIN KIT INCLUDES:
| Part 91022A | Part 91022B | ||
| Solution A: | Acetic Acid 3%, Aqueous | 250 ml | 500 ml |
| Solution B: | Alcian Blue Stain 1%, pH 2.5, Aqueous | 250 ml | 500 ml |
| Solution C: | Periodic Acid 0.5%, Aqueous | 250 ml | 500 ml |
| Solution D: | Schiff Reagent, McManus | 250 ml | 500 ml |
| Solution E: | Hematoxylin Stain, Mayer Modified | 250 ml | 500 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Alcian Blue/PAS Stain Kit procedure is used to differentiate acidic epithelial mucins (sialomucin, sulfomucin), neutral epithelial mucins and stromal (mesenchymal) mucins and is a means of detecting the overall presence of mucins.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
STAINING PROCEDURE:
-
- If necessary, heat dry tissue sections/slides in oven.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Place slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5, Aqueous for 15 minutes.
- Wash slides gently running tap water 1-2 minutes; rinse in distilled water.
- Place in Solution C: Periodic Acid 0.5%, Aqueous for 5 minutes.
- Wash in running tap water for 1-2 minutes; rinse in distilled water.
- Place in Solution D: Schiff Reagent, McManus for 10 minutes.
- Wash in lukewarm tap water for 5-10 minutes.
- Stain lightly in Solution E: Hematoxylin Stain, Mayer Modified for 1 minute.
- Rinse in running tap water for 1-2 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucin | Violet |
| Neutral epithelial mucin | Magenta |
| Glycogen | Magenta |
| Stromal (mesenchymal) mucin | Blue |
| Nuclei | Pale blue |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 173-174.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 150-151.
- Modifications developed by Newcomer Supply Laboratory
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alcian Blue/PAS quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Alcian Blue/PAS Stain Kit: | Part 91022A/B | Individual Stain Solution | |
| Solution A: | Acetic Acid 3%, Aqueous | 250/500 ml | Part 10017 |
| Solution B: | Alcian Blue Stain 1%, pH 2.5 Aqueous | 250/500 ml | Part 1003 |
| Solution C: | Periodic Acid 0.5%, Aqueous | 250/500 ml | Part 13308 |
| Solution D: | Schiff Reagent, McManus | 250/500 ml | Part 1371 |
| Solution E: | Hematoxylin Stain, Mayer Modified | 250/500 ml | Part 1202 |
APPLICATION:
Newcomer Supply Alcian Blue/PAS Control Slides are for the positive histochemical staining and differentiation of acidic epithelial mucins (sialomucin, sulfomucin), stromal (mesenchymal) mucin, neutral mucins and glycogen.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Place slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5 Aqueous for 15 minutes.
- Wash slides in gently running tap water for 1-2 minutes; rinse in distilled water.
- Place in Solution C: Periodic Acid 0.5%, Aqueous for 5 minutes.
- Wash in running tap water for 1-2 minutes; rinse in distilled water.
- Place in Solution D: Schiff Reagent, McManus for 10 minutes.
- Wash in lukewarm tap water for 5-10 minutes.
- Stain lightly in Solution E: Hematoxylin Stain, Mayer Modified for 1 minute.
- Rinse in running tap water for 1-2 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Violet |
| Neutral epithelial mucin | Magenta |
| Glycogen | Magenta |
| Stromal (mesenchymal) mucin | Blue |
| Nuclei | Pale blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 173-174.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 150-151
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 500 ml | 1 Liter | |
| Tartrazine Stain 1.5%, Aqueous | Part 14015A | Part 14015B |
Additionally Needed:
| Gram, Multi-Tissue, Artificial Control Slides OR Gram+ & Gram- Bacteria, Artificial Control Slides |
Part 4256 OR Part 4255 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Crystal Violet Stain 1%, Aqueous, Brown-Hopps | Part 1041 |
| Iodine, Gram, Aqueous | Part 1140 |
| Acetone, ACS | Part 10014 |
| Basic Fuchsin Stain 0.25%, Aqueous | Part 1011 |
| Gallego Solution | Part 1098 |
| Acetone-Xylene 1:1 | Part 10015 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Brown-Hopps Modified Gram Stain with Tartrazine is a modification of the original Gram stain technique. Tartrazine, a synthetic water soluble, lemon yellow colored azo dye, provides a safe alternative to picric acid stains. Tartrazine Stain 1.5%, Aqueous provides yellow background staining, replacing the picric acid-acetone counterstain and the disposable issues associated with picric acid.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- If necessary, heat dry tissue sections/slides in oven
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Stain in Crystal Violet Stain 1%, Aqueous, Brown-Hopps (Part 1041) for 2 minutes.
- Rinse well in distilled water.
- Mordant in Iodine, Gram, Aqueous (Part 1140) for 5 minutes.
- Rinse well in distilled water.
- Blot excess water from slide; decolorize one slide at a time in Acetone (Part 10014) until the blue stops running, 1-2 dips.
- Sections should be very light gray in color.
- Quickly rinse in running tap water.
- Place in Basic Fuchsin Stain 0.25% Aqueous (Part 1011) for 5 minutes.
- Rinse well in running tap water.
- Differentiate sections in Gallego Solution (Part 1098) for 5 minutes.
- Rinse in running tap water. Blot water off slide(s) but not to dryness.
- Proceed with Steps #13 to #16 one slide at a time.
- Place in Tartrazine Stain 1.5%, Aqueous for 1 minute.
- Rinse well in running tap water.
- Dip in Acetone, 1-2 quick dips.
- Dip in Acetone-Xylene 1:1 (Part 10015) for 5 dips.
- Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Gram-positive bacteria | Blue |
| Gram-negative bacteria | Red |
| Nuclei | Red |
| Background tissue | Yellow |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Brown, Robert C., and Howard C. Hopps. “Staining of Bacteria in Tissue Sections: A Reliable Gram Stain Method.” American Journal of Clinical Pathology 60.2 (1973): 234-240.
- Dapson, Janet Crookham, and Richard Dapson. Hazardous Materials in the Histopathology Laboratory: Regulations, Risks, Handling, and Disposal. 4th ed. Battle Creek, MI: Anatech, 2005. 150, 182, 266.
- Mercado, Gene. “Modifying the Modification: How to Redden Shy Gram Negatives.” The Journal of Histotechnology 25.2 (2002): 115-116.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 235.
- Modifications developed by Newcomer Supply Laboratory.
Slotted lid and base for max fluid flow. Large labeling areas on 3 sides. Anterior edge @ 45° angle for manual or instrument labeling. Covers detached and packaged separately in the case.
CaviWipes Surface Disinfection Towelettes are pre-saturated with CaviCide, and are a cleaner and disinfectant in one. The durable, non-woven, non-abrasive towelettes offer quick, easy-to-use, time-saving convenience. They are recommended for use on hard non-porous surfaces and fixtures. CaviWipes Surface Disinfection Towelettes should not be disposed of in the toilet.
CAVIWIPES SURFACE DISINFECTION TOWELETTES FEATURES AND BENEFITS:
- Proven effective against SARS-CoV-2 on hard non-porous surfaces
- Kills TB 3 minutes and MRSA, HIV-1 and HCV in 2 minutes
- Cleaner and disinfectant in one
- Stays fully saturated – designed to help optimize fluid capacity during use
- Convenient, ready-to-use towel and CaviCide in one
- Nonabrasive – for use on hard non-porous surfaces
- Superior surface contact – durable, non-woven towels won’t bunch up during use
QUALIFICATIONS:
CAVIWIPES SURFACE DISINFECTION TOWELETTES LIST OF USES:
- Ambulance equipment surfaces
- Animal care facilities
- Bathrooms
- Correctional facilities
- Daycare Centers
- Dental Offices
- Emergency medical settings
- Emergency vehicles
- Exterior surfaces of anesthesia machines and respiratory therapy equipment
- Health club facilities
- Hospitals
- Infant/child care equipment surfaces
- Interior and exterior surfaces of infant incubators, bassinets
- Isolation areas
- Laboratories
- Laundry rooms
- Neonatal units
- Nursing homes
- Operating rooms
- Ophthalmic and optometric facilities
- Outpatient surgical centers
- Oxygen hoods
- Schools
- Surgical centers
CAVIWIPES SURFACE DISINFECTION TOWELETTES KILL CLAIMS:
3 Minute Efficacy Against
Mycobacterium
- Mycobacterium tuberculosis var:bovis (BCG)
Bacteria
- Pseudomonas aeruginosa
- Salmonella enterica
- Staphylococcus aureus
Fungi
- Trichophyton mentagrophytes
2 Minute Efficacy Against
Multidrug-Resistant Bacteria
- Methicillin Resistant Staphylococcus aureus (MRSA)
- Staphylococcus aureus with reduced susceptibility to vancomycin
- Vancomycin Resistant Enterococcus faecalis (VRE)
Enveloped Viruses
- Hepatitis B Virus (HBV)
- Hepatitis C Virus (HCV)
- Human Immunodeficiency Virus (HIV-1)
- Herpes Simplex Virus (type 1)
- Herpes Simplex Virus (type 2)
- Influenza A2 Virus
- SARS-CoV-2 (COVID-19 Virus)
- Human Coronavirus
STEINER-CHAPMAN MODIFIED SILVER STAIN KIT INCLUDES:
| Part 9172A | Part 9172B | ||
| Solution A: | Zinc Formalin Sensitizer | 250 ml | 500 ml |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | 500 ml |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 175 ml x 2 | 350 ml x 2 |
| Ingredient D: | Hydroquinone, Powder | 5 Grams | 10 Grams |
| Mini Sampling Spoon | |||
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Hydrochloric Acid 5%, Aqueous | Part 12086 (for acid cleaning glassware) |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Acidulated Water pH 4.0-4.1 | Part 10013 |
| Coplin Jar, Plastic | Part 5184 (for microwave modification) |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Steiner-Chapman Modified Silver Stain Kit procedure, with included microwave modification, is used for staining spirochetes, Helicobacter pylori, Legionella pneumophila, other nonfilamentous bacteria and fungus. This modified method eliminates the use of Uranyl Nitrate and its regulatory and disposal requirements.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Preheat Solution A: Zinc Formalin Sensitizer to 60°C.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C.
- Prepare Hydroquinone Working Solution; combine and mix well.
-
- Ingredient D: Hydroquinone, Powder 0.5 gm (or one rounded scoop with reusable mini sampling spoon)
- Distilled water 25 ml
-
-
- Prepare fresh Reducing Solution by combining:
-
- Solution C: Gum Mastic 2.5 %, Alcoholic 15 ml
- Hydroquinone Working Solution (Step #5) 25 ml
- Filter, then add and mix well:
- Solution B: Silver Nitrate 1%, Aqueous 0.6 ml
- Preheat solution in 45°C water bath. Save for Step #16.
-
- Do not preheat solutions if using Microwave Modification.
- Prepare fresh Reducing Solution by combining:
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
-
- See Procedure Note #3.
-
- Wash well in two changes of Acidulated Water pH 4.0-4.1 (Part 10013).
-
- See Procedure Note #4.
-
- Sensitize slides in preheated Solution A: Zinc Formalin Sensitizer (Step #3) in a 60°C water bath or oven for 15 minutes.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
Microwave Modification: See Procedure Note #5.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Zinc Formalin Sensitizer. Microwave at 60°C for 1 minute.
- Remove from microwave; Move to fume hood, cover and incubate for 1 minute with gentle agitation.
-
-
-
- Rinse in two changes of Acidulated Water pH 4.0-4.1 (Part 10013).
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in 60°C water bath or oven for 15 minutes.
Microwave Modification:
-
-
-
- Place sides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave to 70°C for 1 minute.
- Remove from microwave and let stand an additional 90 seconds; agitate occasionally for even heat distribution.
-
-
-
- Rinse well in several changes of distilled water.
-
- Excessive rinsing may cause nuclei to pick up silver.
-
- Dip briefly in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 3 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45ºC water bath for 15-30 minutes with frequent agitation. Examine microscopically after 15 minutes of incubation.
-
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2 minutes until results are achieved.
-
- Rinse well in several changes of distilled water.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 2-4 minutes.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 2 minutes until desired results are achieved.
-
-
-
- Dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Spirochetes | Dark brown to black |
| Helicobacter pylori | Dark brown to black |
| Legionella pneumophila | Dark brown to black |
| Nonfilamentous bacteria and fungus | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The use of Acidulated Water pH 4.0-4.1 rinses (Steps #9 and #11) is recommended for proper tissue pH and enhanced staining.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 238-239.
- Chapman, Clifford, and Lorelei Margeson. “Use of Zinc Formalin as a Sensitizer in Silver Stains for Spirochetes.” The Journal of Histotechnology 2 (1996): 135-138.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTIONS:
| 125 ml | 250 ml | 500 ml | 1 Liter | 4 Liters | |
| Hydrochloric Acid 20%, Aqueous | Part 12087A | Part 12087B | |||
| Schiff Reagent, McManus | Part 1371A | Part 1371E | Part 1371B | Part 1371C | Part 1371D |
Additionally Needed:
| Normal Tonsil Custom Tissue Slides | Part CT39790A |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Light Green SF Yellowish Stain 0.2%, Aqueous | Part 12202 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
The Newcomer Supply Feulgen Reaction procedure is used for the demonstration of DNA (deoxyribonucleic acid) in tissue sections.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
-
-
- See Procedure Note #1.
-
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Prepare Hydrochloric Acid Working Solution; combine and mix well.
-
- Hydrochloric Acid, 20% Aqueous 16 ml
- Distilled Water 24 ml
- Preheat and maintain Hydrochloric Acid Working Solution at 60°C.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #2 and #3.
-
- Hydrolyze sections in preheated Hydrochloric Acid Working Solution (Step #2) at 60°C for 10 minutes.
-
- See Procedure Notes #4 and #5.
-
- Place slides directly in Schiff Reagent, McManus for 45 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Light Green SF Yellowish Stain 0.2%, Aqueous (Part 12202) for 1 minute.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| DNA | Red-purple |
| Nuclei | Red-purple |
| Background | Green |
PROCEDURE NOTES:
-
- Bouin fixed tissue is unsatisfactory for use with Feulgen reaction.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- For optimal results preheat and maintain Hydrochloric Acid Working Solution at 60°C during the hydrolysis process.
- Prolonged exposure to hydrochloric acid may over-hydrolyze sections with poor staining results.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for the clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008.224-225.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-Instructional Text. 4th ed. Chicago: ASCP Press, 2015. 126-127.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 150.
- Modifications developed by Newcomer Supply Laboratory.
FAT, OIL RED O, PROPYLENE GLYCOL STAIN KIT INCLUDES:
| Part 9119A | ||
| Solution A: | Propylene Glycol 100%, ACS | 250 ml |
| Solution B: | Oil Red O Stain, Propylene Glycol | 250 ml |
| Solution C: | Propylene Glycol 85%, Aqueous | 250 ml |
| Solution D: | Hematoxylin Stain, Mayer Modified | 250 ml |
Individual stain solutions may be available for purchase under separate part numbers.
Additionally Needed:
| Formalin 10%, Phosphate Buffered | Part 1090 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Mount-Quick Aqueous | Part 6271A |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Fat, Oil Red O, Propylene Glycol Stain Kit procedure, classified as a physical staining method, is used for identification of fat/lipid in frozen sections.
METHOD:
Fixation: Fresh tissue or formalin fixed unprocessed tissue
-
-
- See Procedure Note #1.
-
Technique: Frozen sections cut at 8-10 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
STAINING PROCEDURE:
-
- Fix frozen section slides in Formalin 10%, Phosphate Buffered (Part 1090) for 1 minute.
-
- See Procedure Note #2.
-
- Rinse sections carefully in two changes of distilled water.
- Blot off excess water and place slides in Solution A: Propylene Glycol 100%, ACS with agitation for 2-5 minutes.
- Place slides directly into Solution B: Oil Red O Stain, Propylene Glycol for 1 hour. Agitate occasionally or place Coplin jar on rotator/shaker with continuous gentle agitation.
-
- See Procedure Notes #3 and #4.
-
- Differentiate in Solution C: Propylene Glycol 85%, Aqueous with agitation for a minimum of 3 minutes.
- Rinse gently in two changes of distilled water.
- Counterstain with Solution D: Hematoxylin Stain, Mayer Modified, for 2-3 minutes.
- Wash gently in several changes of tap water.
- Optional: Blue slides in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- Wash gently in several changes of tap water.
- Blot excess water from slide; coverslip with Mount-Quick Aqueous (Part 6271A) mounting medium.
-
- See Procedure Note #5.
-
- Fix frozen section slides in Formalin 10%, Phosphate Buffered (Part 1090) for 1 minute.
RESULTS:
| Fat: | Bright red |
| Nuclei: | Blue to dark blue |
PROCEDURE NOTES:
-
- To freeze formalin fixed unprocessed tissue:
-
- Place fixed specimen in tissue cassette, wash in running water for 5 minutes.
- Remove tissue and blot excess water from tissue.
- Freeze tissue (fresh or formalin fixed) according to your laboratory protocol.
-
- Frozen formalin fixed tissue does not require additional fixation.
- To decrease staining time; preheat Solution B: Oil Red O Stain, Propylene Glycol in a 60oC oven and incubate for 7-10 minutes.
- If filmy precipitate forms on Solution B: Oil Red O Stain, Propylene Glycol, skim the surface with filter paper before use.
- Use minimal pressure when applying coverslip or fat/lipid staining may be disturbed. To remove trapped air bubbles or to recoverslip;
-
- Soak slide in warm water until coverslip is easily removed.
- Blot excess water from slide.
- Remount with new coverslip and Mount-Quick Aqueous mounting medium.
-
- To freeze formalin fixed unprocessed tissue:
REFERENCES:
-
- Prophet, Edna B., Bob Mills, Jacquelyn Arrington, and Leslie Sobin. Laboratory Methods in Histotechnology. Washington, D.C.: American Registry of Pat 1992.178.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 205.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 500 ml | 1 Liter | |
| Van Gieson Stain | Part 1404A | Part 1404B |
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Van Gieson Stain is a connective tissue stain and/or counterstain that combines acid fuchsin and saturated picric acid, with acid fuchsin selectively staining collagen and osteoid tissue red and the picric acid component staining muscle, elastin, fibrin and cytoplasm yellow.
This stain solution is commonly used in elastic stains, referred to as the Verhoeff-Van Gieson (VVG) technique. Other procedures that use Van Gieson Stain include;
- Bile Stain, Hall’s Method
- Colloidal Iron, Müller-Mowry Stain
- Sulfated Alcian Blue (SAB) Stain
- Van Gieson’s Picric Acid-Fuchsin Stain
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Proceed with selected stain procedure:
- Verhoeff-Van Gieson (VVG) Elastic Stain
- Bile Stain, Hall’s Method
- Colloidal Iron, Müller-Mowry Stain
- Sulfated Alcian Blue (SAB) Stain
- Van Gieson’s Picric Acid-Fuchsin Stain
- Or other appropriate stain procedure
- Counterstain in Van Gieson Stain for 3 to 5 minutes.
- See Procedure Note #3.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Collagen | Red |
| Muscle, elastin, fibrin, cytoplasm | Yellow |
| Other tissue components | Dependent on stain procedure used |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The picric acid element may act as a decolorizer in some procedures.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 146-147.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 166-167.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 189, 196.
- Modifications developed by Newcomer Supply Laboratory.
SET INCLUDES:
| Part 14034A | Part 14034B | ||
| Solution A: | Neutral Red Stain 1%, Alcoholic | 250 ml | 500 ml |
| Solution B: | Fast Green Stain 1%, Alcoholic | 100 ml | 200 ml |
Additionally Needed For Gram Stain, Hucker-Twort:
| Gram, Multi-Tissue, Artificial Control Slides OR Gram+ & Gram- Bacteria, Artificial Control Slides |
Part 4256 OR Part 4255 |
| Crystal Violet-Oxalate Stain, Alcoholic | Part 10422 |
| Iodine, Lugol’s, Aqueous | Part 12092 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Acetone, ACS | Part 10014 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Twort’s Gram Stain Set provides stain solutions for the Gram Stain, Hucker-Twort, a simple, rapid procedure for staining gram-positive and gram-negative bacteria without picric acid. The Twort Stain combines Neutral Red and Fast Green, for clear detection of red gram-negative bacteria with a green counterstain.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Sets are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure. Some solutions in the set may contain extra volumes.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Filter Crystal Violet-Oxalate Stain, Alcoholic (Part 10422) with high quality filter paper.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Stain in freshly filtered Crystal Violet-Oxalate Stain, Alcoholic, (Step #2) for 30 seconds.
- Rinse quickly in distilled water.
- Mordant in Iodine, Lugol’s, Aqueous (Part 12092) for 20 seconds.
- Rinse quickly in distilled water.
- Decolorize individually with Acetone, ACS (Part 10014); 2 quick dips.
-
- Or until tissue remains light gray.
-
- Rinse quickly in distilled water.
- Prepare fresh Twort Stain; combine and mix well.
-
- Solution A: Neutral Red Stain 1%, Alcoholic 9 ml
- Solution B: Fast Green Stain 1%, Alcoholic 3 ml
- Distilled Water 30 ml
- Use within 30 minutes.
-
- Stain in fresh Twort Stain for 2 minutes.
- Rinse quickly in distilled water; carefully blot dry.
- Agitate slides quickly in clean Acetone, ACS to remove excess stain and dehydrate (do not use any alcohols).
-
- The use of alcohols will remove Neutral Red.
-
- Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Gram-positive bacteria | Dark blue |
| Gram-negative bacteria | Red |
| Cytoplasm and red blood cells | Shades of green |
| Nuclei | Red |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Alan Stevens. Theory and Practice of Histological Techniques. 3rd ed. Edinburgh: Churchill Livingstone, 1990. 290-292.
- Culling, C.F.A. Handbook of Histopathological and Histochemical Techniques. 3rd ed. London: Butterworth, 1974. 393-395.
- Twort, F.W., “An Improved Neutral Red, Light Green Double Staining for Animal Parasites, Microorganisms and Tissues”. Journal of State Medicine (1924). 351.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo: Toluidine Blue Stain for Mast Cells
SOLUTION:
| 250 ml | 500 ml | 1 Gallon | |
| Toluidine Blue Stain 0.1%, Aqueous | Part 14027A | Part 14027B | Part 14027D |
Additionally Needed:
| Mast Cell Control Slides OR Mast Cell, Animal Control Slides |
Part 4410 OR Part 4412 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Toluidine Blue Stain for Mast Cells is for the demonstration of mast cells, characterized as cells filled with basophilic granules, associated with inflammation and allergic reactions, which stain metachromatically with toluidine blue.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Toluidine Blue Stain 0.1%, Aqueous for 10 minutes.
- Rinse well in distilled water.
- Dehydrate quickly through two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- See Procedure Note #3.
RESULTS:
| Mast cells | Deep blue-violet |
| Background | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Metachromasia of mast cell granules is stable and staining will be maintained during dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Broome, Michelle and Beth Villarreal. “Differential Staining of Mast Cells with Toluidine Blue”. The Journal of Histotechnology 35.1 (2012): 27-30.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.188.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo: Toluidine Blue Stain for Mohs Technique
SOLUTION:
| 250 ml | 500 ml | 1 Gallon | |
| Toluidine Blue Stain 0.1%, Aqueous | Part 14027A | Part 14027B | Part 14027D |
Additionally Needed:
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Xylene, ACS | Part 1445 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Toluidine Blue Stain for Mohs Technique provides a rapid staining method for Mohs micrographic surgery (MMS), useful when evaluating frozen skin samples for basal cell carcinoma (BCC). Toluidine Blue imparts an identifiable staining pattern if BCC is present that will highlight islands of blue staining basal cell carcinoma and metachromatically stain surrounding mucopolysaccharides/stroma pink.
METHOD:
Fixation: 70% Ethyl Alcohol (Part 10844)
Technique: Frozen sections cut at 5-7 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Fix tissue sections in 70% Ethyl Alcohol for 30-60 seconds.
- See Procedure Note #1.
- Wash well in distilled water.
- Stain slides in Toluidine Blue Stain 0.1%, Aqueous for 30-60 seconds, depending on preference of stain intensity.
- Wash gently in distilled water.
- Dehydrate quickly through one change of 95% ethyl alcohol; 1 quick dip and then two changes 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- See Procedure Note #2.
RESULTS:
| Islands of basal cell carcinoma | Deep blue to purple |
| Surrounding mucopolysaccharides/stroma | Pink to magenta |
| Background | Blue |
| Nuclei | Dark blue |
PROCEDURE NOTES:
- Section thickness and fixation timing may affect staining quality.
- Alcohol will work as a differentiator. Proceed quickly through dehydration steps to maintain Toluidine Blue stain.
- If using a xylene substitute, closely follow the manufacturer’s recommendation for clearing step.
REFERENCES:
- Arnon, Ofer, Ronald Rapini, Adam Mamelak, and Leonard Goldberg. “Mohs Micrographic Surgery: Current Techniques.” IMAJ 12 (2010): 431-35.
- Gross, Kenneth G. Mohs Surgery: Fundamentals and Techniques. St. Louis: Mosby, 1999. 125-138.
- Modifications developed by Newcomer Supply Laboratory.
PRACTICAL APPLICATION OF THIAZINE STAIN SOLUTION
Histology:
-
- Stain for touch preps on fresh tissue during intraoperative procedures (See Reference 1)
- Rapid, inexpensive stain to identify H. pylori in gastrointestinal tissue sections (See Reference 2)
Cytology:
-
- Adequacy assesment of FNA’s (See Reference 3 & 4)
Hematology:
-
- As part of a staining system for blood smears (Click here for Differential Stain Kit Part #9112B)
REFERENCES:
- Yulin Liu M.D., Jan F. Silverman M.D., Charles D. Sturgis M.D., Henry G. Brown M.D., David J. Dabbs M.D. and Stephen S. Raab M.D. Utility of intraoperative consultation touch preparations, Diagnostic Cytopathology Volume 26, Issue 5, pages 329-333, May 2002.
- Skipper, Ray, DeStephano, Don B. A Rapid Stain for Campylobacter pylori in Gastrointestinal Tissue Sections Using Diff-Quik® J of Histotechnology Volume 12 Issue 4. 1989, pp. 303-304.
- Silverman JF, Frable WJ. The use of the Diff-Quik stain in the immediate interpretation of fine-needle aspiration biopsies. Diagon Cytopathology 1990; 6:366-369.
- Rosemary H. Tambouret, MD; Guliz A. Barkan, MD; Daniel F.I. Kurtycz, MD; Vijayalakshmi Padmanabhan, MD Rapid on-site evaluation – how practice varies. CAP Today Volume 128, No. 5, pages 29-31, May 2014
SOLUTION:
| 125 ml | |
| Sudan IV Stain, Herxheimer Alcoholic | Part 1400A |
Additionally Needed:
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Hematoxylin Stain, Mayer Modified | Part 1202 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Mount-Quick Aqueous Mounting Medium | Part 6271A |
APPLICATION:
Newcomer Supply Sudan IV Stain, Herxheimer Alcoholic procedure is used for identification of fat/lipid in frozen sections. Herxheimer method refers to an acetone/alcohol solvent mixture; the acetone component of this solution may dissolve out small amounts of lipid.
Sudan dyes are a group of fat/lipid soluble solvent dyes, also known as lysochromes. These solvent dyes readily stain fat/lipid due to the fact that the dyes are more soluble in lipid than in the solvents from which they are applied.
METHOD:
Fixation: Fresh tissue or formalin fixed unprocessed tissue
- See Procedure Note #1.
Technique: Frozen sections cut at 8 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Fix frozen section slides in Formalin 10%, Phosphate Buffered for 1 minute.
- See Procedure Note #2.
- Rinse sections carefully in two changes of distilled water.
- Rinse in Alcohol, Ethyl Denatured, 70% (Part 10844).
- Stain in Sudan IV Stain, Herxheimer Alcoholic for 10 minutes.
- Keep tightly capped to avoid evaporation.
- Differentiate quickly in Alcohol, Ethyl Denatured, 70% to remove excess stain.
- Wash thoroughly in distilled water.
- Counterstain with Hematoxylin Stain, Mayer Modified (Part 1202) for 2-3 minutes.
- Wash gently in several changes of tap water.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- The use of a bluing agent is optional.
- Wash gently in several changes of tap water.
- Blot excess water from slide; coverslip with Mount-Quick Aqueous Mounting Medium.
- See Procedure Note #3.
RESULTS:
| Fat | Orange/red |
| Nuclei | Blue |
PROCEDURE NOTES:
- To freeze formalin fixed unprocessed tissue:
- Place specimen in tissue cassette; wash in running water for 5 minutes.
- Remove tissue from cassette; blot well, removing all excess water from tissue.
- Freeze tissue according to laboratory protocol.
- Frozen formalin fixed tissue does not require additional formalin fixation.
- Use minimal pressure when applying coverslip or fat/lipid staining may be disturbed. To remove trapped air bubbles or to recoverslip;
- Soak slide in warm water until coverslip is easily removed.
- Blot excess water from slide.
- Remount with new coverslip and Mount-Quick Aqueous Mounting Medium.
REFERENCES:
- Culling, C. F. A. Handbook of Histopathological and Histochemical Techniques: (including Museum Techniques). 3rd ed. London: Butterworth, 1974. 359-362.
- Kiernan, J. A. Histological and Histochemical Methods: Theory and Practice. 3rd ed. London, Ontario: Arnold, 2003. 251-254.
- Lillie, R. D., and Harold Fullmer. Histopathologic Technic and Practical Histochemistry. 4th ed. New York: McGraw-Hill, 1976. 565-568.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo 1: Sudan III Stain, Saturated Alcoholic for Frozen Sections
SOLUTION:
| 500 ml | |
| Sudan III Stain, Saturated Alcoholic | Part 1390A |
Additionally Needed:
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Hematoxylin Stain, Mayer Modified | Part 1202 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Mount-Quick Aqueous Mounting Medium | Part 6271A |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Sudan III Stain, Saturated Alcoholic is used for identification of fat/lipid in frozen tissue sections. Sudan dyes are a group of fat/lipid soluble solvent dyes, also known as lysochromes. These solvent dyes readily stain fat/lipid due to the fact that the dyes are more soluble in lipid than in the solvents from which they are applied.
METHOD:
Fixation: Fresh tissue or formalin fixed unprocessed tissue
- See Procedure Note #1.
Technique: Frozen tissue sections cut at 8 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Fix frozen section slides in Formalin 10%, Phosphate Buffered for 1 minute.
- See Procedure Note #2.
- Rinse sections carefully in two changes of distilled water.
- Rinse in Alcohol, Ethyl Denatured, 70% (Part 10844).
- Stain in Sudan III Stain, Saturated Alcoholic for 10 minutes.
- Keep tightly capped to avoid evaporation.
- Differentiate in Alcohol, Ethyl Denatured, 70%.
- Wash thoroughly in distilled water.
- Counterstain with Hematoxylin Stain, Mayer Modified (Part 1202) for 2-3 minutes.
- Wash gently in several changes of tap water.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- The use of a bluing agent is optional.
- Wash gently in several changes of tap water.
- Blot excess water from slide; coverslip with Mount-Quick Aqueous Mounting Medium.
- See Procedure Note #3.
RESULTS:
| Fat | Orange/red |
| Nuclei | Blue |
PROCEDURE NOTES:
- To freeze formalin fixed unprocessed tissue post:
- Place specimen in tissue cassette, wash in running water for 5 minutes.
- Remove tissue from cassette; blot well, removing all excess water from tissue.
- Freeze tissue according to laboratory protocol.
- Frozen formalin fixed tissue does not require additional formalin fixation.
- Use minimal pressure when applying coverslip or fat/lipid staining may be disturbed. To remove trapped air bubbles or to recoverslip;
- Soak slide in warm water until coverslip is easily removed.
- Blot excess water from slide.
- Remount with new coverslip and Mount-Quick Aqueous Mounting Medium.
REFERENCES:
- Culling, C. F. A. Handbook of Histopathological and Histochemical Techniques: (including Museum Techniques). 3rd ed. London: Butterworth, 1974. 359-362.
- Kiernan, J. A. Histological and Histochemical Methods: Theory and Practice. 3rd ed. London, Ontario: Arnold, 2003. 251-254.
- Modifications developed by Newcomer Supply Laboratory.
Tech Memo 2: Sudan III, Saturated Alcoholic For Fecal Fat
SOLUTION:
| 500 ml | |
| Sudan III Stain, Saturated Alcoholic | Part 1390A |
Additionally Needed:
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Acetic Acid, Glacial, ACS | Part 10010 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Sudan III Stain, Saturated Alcoholic is used for microscopic detection of increased fecal fat levels due to malabsorption. This screening procedure for malabsorption and steatorrhea consists of two parts; the neutral fat stain and the split fat stain for fatty acids.
METHOD:
Fixation: Fresh/unpreserved fecal material
- See Procedure Note #1.
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
STAINING PROCEDURE: METHOD FOR NEUTRAL FATS
- Examine within accepted time, a fresh fecal sample collected, stored and prepared per laboratory protocol.
- See Procedure Note #2.
- Place small aliquot of prepared fecal sample on a clean glass slide; approximately 5mm in diameter.
- Mix two drops of Alcohol, Ethyl Denatured, 95% (Part 10842) with the fecal sample on the slide.
- Add two drops of Sudan III Stain, Saturated Alcoholic to the fecal suspension on the slide; mix well.
- Coverslip and examine microscopically.
RESULTS:
| Neutral fats | Red/orange refractile globules |
STAINING PROCEDURE: SPLIT FAT STAIN FOR FATTY ACIDS
- Prepare in advance 36% Acetic Acid, Glacial; mix well.
- Acetic Acid Glacial, ACS (Part 10010) 9 ml
- Distilled Water 16 ml
- Store at room temperature for up to 1 year.
- Examine within accepted time frame, a fresh fecal sample collected, stored and prepared per laboratory protocol.
- See Procedure Note #2.
- Place small aliquot of prepared fecal sample on a clean glass slide; approximately 5mm in diameter.
- Mix two drops of Alcohol, Ethyl Denatured, 95% (Part 10842) with the fecal sample on the slide.
- Add two drops of Sudan III Stain, Saturated Alcoholic to the fecal suspension on the slide; mix well.
- Add two drops of 36% Acetic Acid Glacial; mix well.
- Place on preheated hot plate (calibrated to 60°C or slightly higher) until bubbles appear. Or hand hold slide over preheated hot plate until bubbles appear; quickly remove slide and reheat two additional times.
- See Procedure Note #3.
- Immediately coverslip and examine microscopically while warm.
RESULTS:
| Fatty acids | Red/orange refractile globules |
PROCEDURE NOTES:
- For optimum results fresh, unpreserved fecal material is required.
- Refer to laboratory protocols and guidelines for proper collection methods, specimen storage requirements and time frame that satisfactory testing results can be obtained.
- Sudan dyes dissolve in lipids at temperatures above the melting point of the lipid or when lipid is in liquid phase. The melting point of lipids in which fatty acid chains are saturated melt above 60°C. Most unsaturated lipids are liquid at room temperature (20°-22°C).
REFERENCES:
- Bauer, John D. Clinical Laboratory Methods. 9th ed. St. Louis: Mosby, 1982. 793-794.
- Fine, Kenneth D., and Frederick Ogunji. “A New Method of Quantitative Fecal Fat Microscopy and Its Correlation With Chemically Measured Fecal Fat Output.” American Journal of Clinical Pathology 113.4 (2000): 528-534.
- Fischbach, Frances, and Marshall Dunning. A Manual of Laboratory and Diagnostic Tests. 8th ed. Lippincott Williams & Wilkins, 2008. 303-304.
- Khouri, M.R., G. Huang, and Y.F. Shiau. “Sudan Stain of Fecal Fat: New Insight Into an Old Test.” Gastroenterology 96.2 (1989): 421-427.
- Kiernan, J. A. Histological and Histochemical Methods: Theory and Practice. 3rd ed. London, Ontario: Arnold, 2003. 251-252, 254.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTIONS:
| 125 ml | 250 ml | |
| Sudan Black B Stain, Propylene Glycol | Part 1401A | Part 1401B |
Additionally Needed:
| Formalin 10%, Phosphate Buffered | Part 1090 |
| Propylene Glycol 100%, ACS | Part 13391 |
| Propylene Glycol 85%, Aqueous | Part 133912 |
| Nuclear Fast Red Stain, Kernechtrot | Part 1255 |
| Mount-Quick Aqueous Mounting Medium | Part 6271A |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Sudan Black B Stain, Propylene Glycol procedure is used for identification of fat/lipid in frozen sections. Sudan black B is a lipid soluble solvent dye that readily stains neutral fats and phospholipids.
Sudan dyes are a group of fat/lipid soluble solvent dyes, also known as lysochromes. These solvent dyes readily stain fat/lipid due to the fact that the dyes are more soluble in lipid than in the solvents from which they are applied.
METHOD:
Fixation: Fresh tissue or formalin fixed unprocessed tissue
-
- See Procedure Note #1.
Technique: Frozen sections cut at 8-10 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Fix frozen section slides in Formalin 10%, Phosphate Buffered (Part 1090) for 1 minute.
- See Procedure Note #2.
- Rinse sections carefully in two changes of distilled water.
- Blot off excess water and dehydrate slides in Propylene Glycol 100%, ACS (Part 13391) for 10-15 minutes.
- Place directly into Sudan Black B Stain, Propylene Glycol for 30 minutes to 1 hour. Agitate occasionally or place Coplin jar on rotator/shaker with continuous gentle agitation.
- See Procedure Note #3.
- Differentiate in Propylene Glycol 85%, Aqueous (Part 133912) for 3 minutes with agitation.
- Rinse gently in distilled water.
- Counterstain in Nuclear Fast Red Stain, Kernechtrot (Part 1255) for 5 minutes.
- Shake solution well before use; do not filter.
- Wash gently in several changes of tap water.
- See Procedure Note #4.
- Blot excess water from slide; coverslip with Mount-Quick Aqueous (Part 6271A) Mounting Medium.
- See Procedure Note #5.
RESULTS:
| Fat | Blue-black |
| Nuclei | Red |
PROCEDURE NOTES:
- To freeze formalin fixed unprocessed tissue:
- Place specimen in tissue cassette, wash in running tap water for 5 minutes.
- Remove tissue from cassette; blot well, removing all excess water from tissue.
- Freeze tissue according to laboratory protocol.
- Frozen formalin fixed tissue does not require additional formalin fixation.
- To decrease staining time; preheat Sudan Black B Stain, Propylene Glycol in a 60°C oven; stain for 3-10 minutes.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in coverslipped sections.
- Use minimal pressure when applying coverslip or fat/lipid staining may be disturbed. To remove trapped air bubbles or to recoverslip;
- Soak slide(s) in warm water until coverslip is easily removed.
- Blot excess water from slide.
- Remount with new coverslip and Mount-Quick Aqueous Mounting Medium.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 192-193.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 185-186.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 464-465.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 204-205.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 500 ml | |
| Safranin O Stain 0.25%, Aqueous | Part 1360A |
Additionally Needed:
| Crystal Violet Stain 1%, Aqueous, Brown-Hopps | Part 1041 |
| Iodine, Gram, Aqueous | Part 1140 |
| Acetone, ACS | Part 10014 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Safranin O Stain 0.25%, Aqueous provides the preferred counterstain for Gram staining of microbiology smears. The Gram Stain technique, is used for differential staining of gram-positive and gram-negative bacteria in both smears and tissue sections.
METHOD:
Technique: Flat staining rack method.
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
STAINING PROCEDURE:
- Prepare within an accepted time frame, a well-made smear(s) per laboratory protocol, with a focus on uniform distribution of material.
- Allow slides to thoroughly air-dry before staining.
- Fix slides according to laboratory protocol.
- See Procedure Note #1.
- Place slides on flat staining rack suspended over sink.
- See Procedure Note #2.
- Flood fixed smears with Crystal Violet Stain 1%, Aqueous, Brown-Hopps (Part 1041) for 45-60 seconds.
- Drain off Crystal Violet Stain and rinse well in distilled water.
- Mordant smears in Iodine, Gram, Aqueous (Part 1140) for 45-60 seconds.
- Rinse well in running tap water to remove excess iodine.
- Blot one slide at a time and individually decolorize in Acetone (Part 10014) until color stops running off the smear; 5-10 seconds.
- See Procedure Note #3.
- Quickly rinse in distilled water to remove excess acetone.
- Counterstain in Safranin O Stain 0.25%, Aqueous for 30-60 seconds.
- See Procedure Note #4.
- Wash well in distilled water.
- Allow slides to air-dry.
- Examine microscopically with oil immersion.
RESULTS:
| Gram-positive bacteria | Blue to blue/black |
| Gram-negative bacteria | Pink to red |
| Background | Yellow |
PROCEDURE NOTES:
- Gentle heat or methanol are both accepted methods of smear fixation.
- Do not allow smears to dry out at any point during staining procedure.
- To decolorize slower, dip slides in Alcohol, Ethyl Denatured, 95% (Part 10842) in Coplin jar for 10-60 seconds or until color stops running off the smear.
- For enhanced counterstaining;
- Safranin O Stain 0.25%, Aqueous 10 ml
- 95% Ethyl Alcohol (Part 10842) 1 ml
- Combine; mix well and stain for 30-60 seconds.
REFERENCES:
- Kidd, Larry. “Histology vs. Microbiology – The Gram Stain the Easy Way.” The Journal of Histotechnology 7.2 (1984): 85-86.
- Lillie, R. D., and Harold Fullmer. Histopathologic Technic and Practical Histochemistry. 4th ed. New York: McGraw-Hill, 1976. 726-727.
- McPherson, Richard and Matthew Pincus. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia: Elsevier Saunders, 2011. 1080.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 235.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 250 ml | 500 ml | |
| Oil Red O Stain, Propylene Glycol | Part 12772A | Part 12772B |
Additionally Needed:
| Formalin 10%, Phosphate Buffered | Part 1090 |
| Propylene Glycol 100%, ACS | Part 13391 |
| Propylene Glycol 85%, Aqueous | Part 133912 |
| Hematoxylin Stain, Mayer Modified | Part 1202 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Mount-Quick Aqueous | Part 6271A |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Oil Red O Stain, Propylene Glycol procedure is classified as a physical staining method and is used for identification of fat/lipid in frozen sections.
METHOD:
Fixation: Fresh tissue or formalin fixed unprocessed tissue
Technique: Frozen sections cut at 8-10 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
- Fix frozen section slides in Formalin 10%, Phosphate Buffered (Part 1090) for 1 minute.
- See Procedure Note #2.
- Rinse sections carefully in two changes of distilled water.
- Blot excess water and place slides in Propylene Glycol 100%, ACS (Part 13391) for 2-5 minutes.
- Place directly into Oil Red O Stain, Propylene Glycol for 1 hour. Agitate occasionally or place Coplin jar on rotator/shaker with continuous gentle agitation.
- See Procedure Notes #3 and #4.
- Differentiate in Propylene Glycol 85%, Aqueous (Part 133912) with agitation for a minimum of 3 minutes.
- Rinse gently in two changes of distilled water.
- Counterstain with Hematoxylin Stain, Mayer Modified (Part 1202) for 2-3 minutes.
- Wash gently in several changes of tap water.
- Blue in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- The use of a bluing agent is optional.
- Wash gently in several changes of tap water.
- Blot excess water from slide; coverslip with Mount-Quick Aqueous (6271A) mounting medium.
- See Procedure Note #5.
RESULTS:
| Fat | Bright red |
| Nuclei | Blue to dark blue |
PROCEDURE NOTES:
- To freeze formalin fixed unprocessed tissue:
- Place specimen in tissue cassette, wash in running tap water for 5 minutes.
- Remove tissue from cassette; blot well, removing all excess water from tissue.
- Freeze tissue according to laboratory protocol.
- Frozen formalin fixed tissue does not require additional formalin fixation.
- To decrease staining time; preheat Oil Red O Stain, Propylene Glycol in a 60°C oven; stain for 7-10 minutes.
- If a filmy precipitate develops in Oil Red O Stain, Propylene Glycol, filter with coarse filter paper.
- Use minimal pressure when applying coverslip or fat/lipid staining may be disturbed. To remove trapped air bubbles or to recoverslip;
- Soak slide in warm water until coverslip is easily removed.
- Blot excess water from slide.
- Remount with new coverslip and Mount-Quick Aqueous mounting medium.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 184-186.
- Prophet, Edna B., Bob Mills, Jacquelyn Arrington, and Leslie Sobin. Laboratory Methods in Histotechnology. Washington, D.C.: American Registry of Pathology. 1992.178.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 205.
- Modifications developed by Newcomer Supply Laboratory.
SET INCLUDES:
| Part 1142A | Part 1142B | ||
| Solution A: | Silver Nitrate | 500 ml | 1000 ml |
| Solution B: | Methenamine Borate | 500 ml | 1000 ml |
Additionally Needed For Fungus Stain, Grocott Methenamine Silver, GMS:
| Fungus, GMS, Multi-Tissue, Artificial Control Slides | Part 4235 |
| Hydrochloric Acid 5%, Aqueous | Part 12086 (for acid cleaning glassware) |
| Chromic Acid 5%, Aqueous | Part 10341 |
| Sodium Bisulfite 1%, Aqueous | Part 13821 |
| Gold Chloride 0.1%, Aqueous | Part 11285 |
| Sodium Thiosulfate 2%, Aqueous | Part 13888 |
| Light Green SF Yellowish Stain 0.02%, Aqueous | Part 12204 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Coplin Jar, Plastic | Part 5184 (for microwave modification) |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Grocott Methenamine Silver Set, GMS with included microwave modifications, provides the silver solutions for the Fungus GMS stain procedure. This is one of the best staining methods to demonstrate a variety of fungal organisms including: Pneumocystis, Aspergillus, Blastomyces, Candida and Histoplasma.
When staining for Pneumocystis with other fungal organisms, running a separate control specific for Pneumocystis (Part 4556) is recommended.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Sets are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure. Some solutions in the set may contain extra volumes.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well:
-
- Solution A: Silver Nitrate 20 ml
- Solution B: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath 20 minutes before use.
-
- Maintain solution between 45°C-60°C to minimize precipitate.
- Save for use in Step #10.
- Do not preheat if using Microwave Modification; Step 11.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #3 and #4.
-
- Oxidize in Chromic Acid 5%, Aqueous (Part 10341) for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #5.
-
-
-
- Oxidize slides in a plastic Coplin jar with Chromic Acid 5%, Aqueous. Microwave for 1 minute and 20 seconds at 60°C.
-
-
-
- Wash well in running tap water; rinse in distilled water.
- Place in Sodium Bisulfite 1%, Aqueous (Part 13821) for 1 minute.
- Wash for 5 minutes in running tap water; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Pneumocystis may take longer to stain than other fungus.
- Staining at room temperature will require longer incubation.
-
- Microwave Modification:
-
- Incubate slides in a plastic Coplin jar with Silver-Methenamine Working Solution (Step #3). Microwave for 1 minute at 70°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 2-3 minute intervals.
-
- Rinse in three to four changes of distilled water.
-
- Do not use tap water at this step.
-
- Tone in Gold Chloride 0.1%, Aqueous (Part 11285) until sections turn gray; 20 seconds to 1 minute.
- Rinse well in distilled water.
- Remove unreduced silver in Sodium Thiosulfate 2%, Aqueous (Part 13888) for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Light Green SF Yellowish Stain 0.02%, Aqueous (Part 12204) for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Fungi | Crisp black cell walls with visible internal structures |
| Background | Green |
| Mucin | Taupe to dark gray |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped, or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 239-243.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 4.3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 500 ml | 1 Liter | |
| Giemsa Stock Stain | Part 1120A | Part 1120B |
Additionally Needed:
| Alcohol, Methanol Anhydrous, ACS | Part 12236 |
| Phosphate Buffer, pH 7.0 | Part 1331 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Giemsa Stain is a simple one-step method designed to demonstrate differential staining of cells types in peripheral blood smears and bone marrow smears/films as well as a method for detecting rickettsia, bacteria and parasites.
METHOD:
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
STAINING PROCEDURE:
Prepare within an accepted time frame, a well-made blood smear or bone marrow smear/film per your laboratories protocol, with a focus on uniform cell distribution.
- Proceed with either the thin or thick smear/film staining method.
Thin Smear/Film Staining Method: See Procedure Notes #1 and #2.
- Allow smear to thoroughly air-dry prior to staining.
- Fix smear in Methanol: 1-2 minutes.
- Air-dry slides in a vertical position.
- Prepare fresh 1:20 Working Giemsa Stain; combine and mix well.
- Giemsa Stock Stain 2 ml
- Phosphate Buffer, pH 7.0 (Part 1331) 40 ml
- Stain in Working Giemsa Stain for 20-30 minutes.
- Rinse briefly in Phosphate Buffer, pH 7.0 or distilled water.
- Air-dry slides in a vertical position.
- If coverslip is preferred, allow slides to air-dry; coverslip with compatible mounting medium.
Thick Smear/Film Staining Method: See Procedure Notes #1 and #2.
- Allow smear to thoroughly air-dry prior to staining; several hours or overnight.
- Proceed directly to stain; do not place in fixative.
- Prepare fresh 1:50 Working Giemsa Stain; combine and mix well.
- Giemsa Stock Stain 1 ml
- Phosphate Buffer, pH 7.0 (Part 1331) 50 ml
- Stain in Working Giemsa Stain for 50 minutes.
- Rinse briefly in Phosphate Buffer, pH 7.0 or distilled water.
- Air-dry slides in a vertical position.
- If coverslip is preferred, allow slides to air-dry; coverslip with compatible mounting medium.
RESULTS:
| Erythrocytes | Orange – pink to rose |
| Platelets | Red to purple granules with blue halo |
Granulocytes
| Neutrophils | Nucleus – Dark blue to violet |
| Cytoplasm – Pink | |
| Granules – Purple to lilac | |
| Eosinophils | Nucleus – Blue |
| Granules – Orange to pink | |
| Basophils | Nucleus – Deep blue to violet |
| Granules – Deep blue to violet |
Mononuclear Cells
| Lymphocytes | Nuclei – Deep blue to violet |
| Cytoplasm – Light blue | |
| Monocytes | Nuclei – Light blue/purple |
| Cytoplasm – Pale gray/blue | |
| Mast cells | Nuclei – Deep blue to violet |
| Granules – Deep blue-violet | |
| Malarial parasites | Nucleus – Red chromatin dot |
| Cytoplasm – Blue | |
| Rickettsia | Bluish purple |
| Bacteria | Blue |
PROCEDURE NOTES:
- The timings provided are suggested ranges. Optimal staining times will depend upon smear/film thickness and preference of stain intensity.
- Smears/films containing primarily normal cell populations require minimum staining time.
- Immature cells may require a longer staining time.
- Bone marrow smears/films may require a longer staining time.
REFERENCES:
- Bailey, W. Robert, and Elvyn Scott. Diagnostic Microbiology. 4th ed. St Louis: C. V. Mosby Company, 1974. 394.
- Garcia, Lynne Shore. Diagnostic Medical Parasitology. 5th ed. Washington DC: ASM Press, 2007. 888-889.
- McPherson, Richard and Matthew Pincus. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia: Elsevier Saunders, 2011. 522-531.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 250 ml | 500 ml | |
| Fouchet Reagent | Part 1095A | Part 1095B |
Additionally Needed:
| Bile Control Slides | Part 4060 |
| Van Gieson Stain | Part 1404 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Bile Stain, Hall’s Method is for the demonstration of bile (bilirubin) substances in tissue sections and to distinguish bile pigments from other tissue pigments. The acidity of Fouchet Reagent works to oxidize bilirubin to biliverdin, resulting in a green color development with the Van Gieson Stain serving as a complimentary counterstain.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- Filter Fouchet Reagent with Grade 1 filter paper prior to use.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in freshly filtered Fouchet Reagent for 5 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Stain sections in Van Gieson Stain (Part 1404) for 5 minutes.
- Rinse quickly in 95% ethyl alcohol.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Bile/bilirubin | Emerald green to olive drab |
| Connective tissue | Pink to red |
| Background | Yellow |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 268-269.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 219.
- Modifications developed by Newcomer Supply Laboratory.
Technical Memo 1: Ziehl-Neelsen for AFB Stain
SOLUTION:
| 250 ml | 500 ml | 1 Liter | |
| Carbol Fuchsin Stain, Ziehl-Neelsen | Part 1030A | Part 1030B | Part 1030C |
Additionally Needed for AFB Stain, Ziehl-Neelsen:
| Acid Fast Bacteria (AFB) Control Slides | Part 4011 |
| Acid Alcohol 1% | Part 10011 |
| Light Green SF Yellowish Stain .1%, Aqueous OR Methylene Blue Stain 0.14%, Alcoholic |
Part 12203 OR Part 12401 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Carbol Fuchsin Stain, Ziehl-Neelsen, a crucial element in the AFB Stain, Ziehl-Neelsen is used to demonstrate the presence of acid-fast mycobacteria in tissue sections. Acid-fastness is a physical property of certain bacteria and cellular structures. Carbol Fuchsin Stain, Ziehl-Neelsen, combines phenol and basic fuchsin that works to permeate the lipoid capsule of acid-fast organisms and renders them resistant to acid alcohol decolorization.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- Filter Carbol Fuchsin Stain, Ziehl-Neelsen with filter paper whenever a thick sheen develops on solution surface.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Stain in Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature. Keep solution covered.
- See Procedure Note #3.
- Rinse in running tap water for 2 to 3 minutes.
- Differentiate in Acid Alcohol 1% (Part 10011) until color no longer runs off the slide and sections are pale pink; 3 to 10 rapid dips.
- Wash in running tap water 3 to 5 minutes; rinse in distilled water.
- Counterstain with 3-6 dips in counterstain of choice;
- Light Green SF Yellowish Stain 0.1%, Aqueous (Part 12203)
- Methylene Blue Stain 0.14%, Alcoholic (Part 12401). Do not overstain; sections should be pale blue.
- Rinse slides:
- Light Green SF Yellowish counterstain; rinse with one quick dip in distilled water or proceed directly to Step #10 without a distilled water rinse.
- Methylene Blue counterstain; rinse in running tap water for 1 minute; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid-fast bacilli | Bright red |
| Background | Green (with Light Green SF Yellowish counterstain) |
| Background | Pale blue (with Methylene Blue counterstain) |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Sections can remain in Carbol Fuchsin Stain, Ziehl-Neelsen for up to 60 minutes without adverse effect. Additional differentiation may be required in Step #6.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 218-220.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.
Technical Memo 2: Ziehl-Neelsen for AFB, Fite Stain
SOLUTION:
| 250 ml | 500 ml | 1 Liter | |
| Carbol Fuchsin Stain, Ziehl-Neelsen | Part 1030A | Part 1030B | Part 1030C |
Additionally Needed for AFB Stain, Fite:
| Fite Stain, Nocardia Sp. Control Slides OR Abnormal Animal Spleen Custom Tissue Slides |
Part 4215 OR Part CT28730A |
| Xylene/Peanut Oil, 2:1 | Part 1449 |
| Acid Alcohol 1% | Part 10011 |
| Light Green SF Yellowish Stain 0.1%, Aqueous OR Methylene Blue Stain 0.5%, Aqueous |
Part 12203 OR Part 12402 |
| Xylene, ACS | Part 1445 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Carbol Fuchsin Stain, Ziehl-Neelsen, a crucial element in the AFB Stain, Fite is used to detect the presence of either Nocardia sp. or Mycobacterium leprae sp. (causative agent of leprosy) in tissue sections. Minor procedural variations are included for detection of either organism.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin Sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- Filter Carbol Fuchsin Stain, Ziehl-Neelsen with high quality filter paper.
- If staining for Nocardia sp., prepare Diluted Acid Alcohol Solution:
- Acid Alcohol 1% (Part 10011) 20 ml
- Distilled water 20 ml
STAINING PROCEDURE:
- Deparaffinize slides in Xylene/Peanut Oil, 2:1 (Part 1449), two changes for 10 minutes each.
- See Procedure Note #1
- Drain slides, wipe off excess oil, and blot to opacity taking care to remove residual oil.
- See Procedure Note #2.
- Stain in freshly filtered Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature.
- Rinse well in distilled water.
- Differentiation:
- For Nocardia sp.: Differentiate slides individually in Diluted Acid Alcohol Solution (Step #3) until background is pale pink; 10-20 dips. Quickly rinse in distilled water and check microscopically for correct differentiation.
- For Mycobacterium leprae sp.: Differentiate slides individually in Acid Alcohol 1% (Part 10011) until sections are light pink; 5-10 dips.
- Rinse well in distilled water.
- Counterstain with 5-10 dips in counterstain of choice;
- Light Green SF Yellowish Stain 0.1%, Aqueous (Part 12203).
- Methylene Blue Stain 0.5%, Aqueous (Part 12402). Do not overstain; sections should be pale blue.
- Rinse slides:
- Light Green SF Yellowish counterstain; rinse in distilled water.
- Methylene Blue counterstain; wash in running tap water, rinse in distilled.
- Blot excess water from slide and air-dry or oven-dry completely.
- Dip dried slides in xylene and coverslip with compatible mounting medium.
RESULTS:
| Acid-fast bacilli and Mycobacterium leprae sp. | Red |
| Nocardia sp. | Red |
| Background | Green (with Light Green SF Yellowish counterstain) |
| Background | Pale blue (with Methylene Blue counterstain) |
PROCEDURE NOTES:
- Acid-fastness of leprosy organisms is enhanced when the waxy capsule is protected by the mixture of xylene-peanut oil and avoidance of dehydrating solutions.
- It is important to blot well; residual oil may produce staining artifact.
- A small percentage of Nocardia sp. organisms may resist taking the red stain and remain green (or blue, depending upon counterstain used) due to growth phase of the individual organism.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for coverslipping step.
REFERENCES:
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 220-221.
- Fite, George, P.J. Cambre and M.H. Turner. “Procedure for Demonstrating Lepra Bacilli in Paraffin Sections”. Archives of Pathology 43 (1947). 624-625.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining thyroid and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PAX8 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply PAX8 (paired-box gene 8) Control Slides are for the positive immunohistochemical staining of PAX8, expressed in thyroid, renal cell, endometroid and ovarian carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply PAX8 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| PAX8 positive expression | Brown nuclear staining |
| Myometrium | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque PAX8 (EP298) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque PAX8 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PAX5 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply PAX5 (paired-box gene 5) Control Slides are for the positive immunohistochemical staining of PAX5, a transcription factor expressed throughout B-cell maturation and in most B-cell malignancies such as Hodgkin’s lymphoma.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply PAX5 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| PAX5 positive expression | Brown nuclear staining |
| Adipose | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque PAX-5 (SP34) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque PAX-5 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Factor XIIIa quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Factor XIIIa (fibrin stabilizing factor) Control Slides are for the positive immunohistochemical staining of Factor XIIIa, a blood coagulation enzyme that crosslinks fibrin. Factor XIIIa is used as a marker for fibro-histiocytic proliferations, dermal dendritic cells and to differentiate dermatofibroma from dermatofibrosarcoma.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Factor XIIIa primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Factor XIIIa positive expression | Brown cytoplasmic staining |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Factor XIIIa (EP3372) is the concentrated primary antibody used. Dilute primary antibody to 1/300 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Factor XIIIa Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining kidney and negative staining thyroid.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Epithelial Membrane Antigen quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Epithelial Membrane Antigen (EMA) Control Slides are for the positive immunohistochemical staining of EMA, a marker of epithelial cells expressed in a variety of normal and neoplastic epithelia, including mammary and squamous epithelium and sweat glands. EMA is also noted to be expressed in a number of malignant lymphomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply EMA primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| EMA positive expression | Brown cytoplasmic & membrane staining |
| Thyroid | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque EMA (E29) is the concentrated primary antibody used. Dilute primary antibody to a 1/1200 dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque EMA Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.







