PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Bile Stain, Hall’s Method quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Bile Stain, Hall’s Method: | Individual Stain Solution |
| Fouchet Reagent | Part 1095 |
| Van Gieson Stain | Part 1404 |
APPLICATION:
Newcomer Supply Bile Control Slides are for the positive histochemical staining of bile (bilirubin) substances in tissue sections and to distinguish bile pigments from other tissue pigments.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Filter Fouchet Reagent with high quality filter paper prior to use.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in freshly filtered Fouchet Reagent for 5 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Stain sections in Van Gieson Stain for 5 minutes.
- Rinse slides quickly in 95% ethyl alcohol.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Bile | Emerald green to olive drab |
| Connective tissue | Pink to red |
| Background | Yellow |
PROCEDURE NOTES:
- Drain staining slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 268-269.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 219.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining brain.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 8 microns on Superfrost™ Plus slides.
Quality Control Stain: Bielschowsky quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Bielschowsky, Lester King Modified Stain Kit: | Part 9154A | Individual Stain Solution | |
| Solution A: | Silver Nitrate 20%, Aqueous | 250 ml | Part 13807 |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 100 ml | Part 1006 |
| Solution C: | Developer | 25 ml | |
| Solution D: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
APPLICATION:
Newcomer Supply Bielschowsky Control Slides are for the positive histochemical staining of nerve fibers, neurofibrils/tangles, senile plaques and axons, instrumental in the diagnosis of Alzheimer’s disease and other neurological disorders.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Preheat Coplin jar of Solution A: Silver Nitrate 20%, Aqueous in water bath to 37°C.
- Preheat two Coplin jars of distilled water in 37°C water bath.
- Save for slide rinsing/holding in Steps #7 and #10.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Place slides in preheated Solution A: Silver Nitrate 20%, Aqueous (Step #3) for 15 minutes.
- See Procedure Note #5.
- Remove slides from Solution A: Silver Nitrate 20%, Aqueous and hold slides in warmed distilled water.
- Save Silver Nitrate 20%, Aqueous for Step #8.
- Add Solution B: Ammonium Hydroxide 28-30%, ACS drop by drop in saved Silver Nitrate 20%, Aqueous swirling until precipitate disappears. Do not go past this point.
- Approximately 10 ml of Ammonium Hydroxide 28-30%, ACS will be required.
- Place slides back into the Silver Nitrate Solution with added Ammonium Hydroxide in water bath at 37°C for 10 minutes.
- Remove slides and hold in preheated distilled water.
- Save Ammoniacal Silver Solution for Step #11.
- Add 1 drop of Solution C: Developer to the saved Ammoniacal Silver Solution with swirling motion.
- Return slides to Ammoniacal Silver Solution with added Developer, in 37°C water bath for 5-15 minutes; average time of 6 minutes.
- Check slides microscopically at 3 minutes for development of neurons to dark brown.
- Follow with checks at 1 minute intervals to avoid silver over-development.
- Rinse thoroughly in distilled water for 5 minutes.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous; 5 minutes.
- Rinse thoroughly in tap water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Senile plaques, neurofibrils/tangles | Dark brown to black |
| Neurons | Dark brown |
| White and gray matter | Yellowish brown |
| Nerve fibers, axons | Brown to black |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with silver solutions to prevent precipitation of silver salts. No metals of any kind should come in contact with silver solutions. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- A maximum of 8 slides per 40 ml of Solution A: Silver Nitrate 20%, Aqueous is recommended for proper silver development
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 196-199.
- King, Lester. “The Impregnation of Neurofibrils”. Yale Journal of Biology and Medicine 14.1 (1941). 59-68.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Jones Basement Membrane quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Jones Basement Membrane Stain Kit | Part 9167A | Individual Stain Solution | |
| Solution A: | Methenamine 3%, Aqueous | 250 ml | Part 12239 |
| Solution B: | Silver Nitrate 5%, Aqueous | 50 ml | Part 13805 |
| Solution C: | Sodium Borate 5%, Aqueous | 50 ml | Part 13826 |
| Solution D: | Periodic Acid 0.5%, Aqueous | 250 ml | Part 13308 |
| Solution E: | Gold Chloride 0.25%, Aqueous | 250 ml | Part 11287 |
| Solution F: | Sodium Thiosulfate 2.5%, Aqueous | 250 ml | Part 13889 |
| Solution G: | Light Green SF Yellowish Stain 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
The Newcomer Supply Basement Membrane Control Slides are for the positive histochemical staining of basement membranes in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Prepare Silver-Methenamine Working Solution; combine and mix well:
- Solution A: Methenamine 3%, Aqueous 40 ml
- Solution B: Silver Nitrate 5%, Aqueous 2 ml
- Solution C: Sodium Borate 5%, Aqueous 4 ml
- Proceed to Step #10 for Microwave Modification.
- Preheat Silver-Methenamine Working Solution to 45°C-60°C.
- See Procedure Notes #3 and #4.
- Do not preheat solution if using Microwave Modification.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #5 and #6.
- Place in Solution D: Periodic Acid 0.5%, Aqueous for 15 minutes.
- Wash in gently running tap water for 5 minutes; rinse in distilled water.
- Place slides in preheated Silver-Methenamine Working Solution and incubate in 45°C-60°C oven or water bath, or bench top/room temperature for 12-18 minutes until sections appear paper-bag brown.
- Periodically remove the control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Basement membranes should be dark brown. If the tissue structures are not sufficiently dark, place slides back in heated silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation times.
- Microwave Modification: See Procedure Note #7.
- Place sides in a plastic Coplin jar containing prepared Silver-Methenamine Working Solution (Step #3) and microwave at 70°C for 3 minutes.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to heated Silver-Methenamine Working Solution.
- Rinse in three changes of distilled water.
- Tone sections in Solution E: Gold Chloride 0.25%, Aqueous for 1 minute.
- Rinse well in three changes of distilled water.
- Place in Solution F: Sodium Thiosulfate 2.5%, Aqueous; 2 minutes.
- Wash in gently running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.1%, Aqueous for 1 minute.
- Quickly rinse slides in two changes of distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Kidney glomerular basement membranes | Black |
| Intra-glomerular deposits | Black |
| Reticular Fibers | Black |
| Nuclei | Outlined in black |
| Background | Light Green |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with silver solutions to prevent precipitation of silver salts. No metals of any kind should come in contact with silver solutions. Only glass thermometers should be used.
- Preheating Silver-Methenamine Working Solution to 45°C-60°C prior to incubation is suggested for timely silver development. A water bath or oven can be used for preheating. Begin preheating the silver solution approximately 20-30 minutes before use.
- Staining at higher temperatures will cause the development reaction to happen faster, but may cause precipitate to form in the working silver solution and deposit on slides. Maintaining the silver solution between 45°C-60°C will help to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 97-99.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 187-188.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alcian Blue pH 2.5 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs. .
CONTROL SLIDE VALIDATION:
| With Alcian Blue, 1%, pH 2.5 Stain Kit: | Part 9102A/B | Individual Stain Solution | |
| Solution A: | Acetic Acid 3%, Aqueous | 250/500 ml | Part 10017 |
| Solution B: | Alcian Blue Stain 1%, pH 2.5 Aqueous | 250/500 ml | Part 1003 |
| Solution C: | Nuclear Fast Red Stain, Kernechtrot | 250/500 ml | Part 1255 |
APPLICATION:
Newcomer Supply Alcian Blue pH 2.5, Goblet Cell Control Slides are for the positive histochemical staining of goblet cells as well as other acid epithelial mucins (sialomucin, sulfomucin). Goblet cells normally line the small intestine but are an abnormal finding in Barrett’s esophagus.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Move slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5 Aqueous. Stain for 30 minutes at room temperature or for 15 minutes in a 37°C water bath.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #3.
- Counterstain in Solution C: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #4
- Dehydrate quickly through two changes of 95% ethyl alcohol and two changes of 100% ethyl alcohol. Clear in three xylene changes, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Goblet cells | Bright blue |
| Acid epithelial mucin | Blue |
| Nuclei | Pink-red |
| Cytoplasm | Pale pink |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- A brief dip in Solution A: Acetic Acid 3%, Aqueous from Step #3 can be added before water rinses to remove excess Alcian Blue Solution if needed.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 145-148.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 172-175.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Fontana Masson quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fontana Masson Stain Kit: | Part 9105A | Individual Stain Solution | |
| Solution A: | Silver Nitrate 10%, Aqueous | 250 ml | Part 13806 |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 250 ml | Part 1006 |
| Solution C: | Gold Chloride 0.2%, Aqueous | 250 ml | Part 11286 |
| Solution D: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution E: | Nuclear Fast Red Stain, Kernechtrot | 250 ml | Part 1255 |
APPLICATION:
Newcomer Supply Argentaffin Control Slides are for the positive histochemical staining of argentaffin substances in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Prepare Fontana Silver Working Solution (diamine silver) in an acid cleaned Erlenmeyer flask:
- Solution A: Silver Nitrate 10%, Aqueous; 25 ml
- Add Solution B: Ammonium Hydroxide 28-30%, ACS drop by drop, mix with swirling motion until solution clouds, then clears. Use caution to not add too much Solution B: Ammonium Hydroxide 28-30%, ACS.
- Add more Solution A: Silver Nitrate 10%, Aqueous drop by drop until clear solution becomes slightly cloudy.
- Let solution stand for 2-4 hours before use.
- For use; after standing, filter the solution. Combine 20 ml of this filtered diamine silver solution with 40 ml of distilled water; 60 ml total.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Immerse slides in the Fontana Silver Working Solution (Step #3) in a 45°C to 60°C water bath for 1 hour.
- Check slides microscopically; remove control, rinse in warm distilled water. Confirm that reaction is complete when granules are dark brown and background is colorless.
- Return to heated Fontana Silver Working Solution for longer incubation if indicated.
- Rinse well in three changes of distilled water.
- Immerse in Solution C: Gold Chloride 0.2%, Aqueous; 10 minutes.
- Rinse well in distilled water.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous; 5 minutes.
- Rinse well in distilled water.
- Counterstain in Solution E: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #5.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Melanin and argentaffin granules | Black |
| Nuclei | Pink-red |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 286-287.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 276-277.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 8 microns on Superfrost™ Plus slides.
Quality Control Stain: Bennhold Congo Red quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Amyloid, Bennhold Congo Red Stain Kit: | Part 9103A | Individual Stain Solution | |
| Solution A: | Congo Red Stain 1%, Aqueous | 250 ml | Part 1038 |
| Solution B: | Alkaline Alcohol | 250 ml | Part 1038 |
| Solution C: | Hematoxylin Stain, Mayer Modified | 250 ml | Part 1202 |
APPLICATION:
Newcomer Supply Amyloid, Animal Control Slides are for the positive histochemical staining of extraneous protein deposits in amyloidosis.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Congo Red Stain 1%, Aqueous for 1 hour.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar (Part 5184) containing Solution A: Congo Red Stain 1%, Aqueous and microwave at 70°C for 3 minutes.
- Rinse in two to three changes of tap water; rinse in distilled water.
- Differentiate in Solution B: Alkaline Alcohol, 5 to 30 seconds, agitating constantly until slide background is cleared of Solution A: Congo Red Stain 1%, Aqueous.
- Rinse in two to three changes of tap water; rinse in distilled water.
- Counterstain with Solution C: Hematoxylin Stain, Mayer Modified, 3 to 5 minutes, depending on preference of nuclear stain intensity.
- Wash in running tap water for 5 to 10 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Light Field Microscopy: | ||
| Amyloid | Pink to red | |
| Nuclei | Blue | |
| Polarized Light: | ||
| Amyloid fluorescence | Apple green | |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- For optimal results cut sections at 8-10 microns to provide more intense staining and allow smaller amyloid deposits to be identified. Thinner sections may show faint staining and sections thicker than 8-10 microns may display yellow birefringence.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 366-367.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 177-178.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining Barrett’s esophagus.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alcian Blue pH 2.5 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Alcian Blue 1%, pH 2.5 Stain Kit: | Part 9102A/B | Individual Stain Solution | |
| Solution A: | Acetic Acid 3%, Aqueous | 250/500 ml | Part 10017 |
| Solution B: | Alcian Blue Stain 1%, pH 2.5 Aqueous | 250/500 ml | Part 1003 |
| Solution C: | Nuclear Fast Red Stain, Kernechtrot | 250/500 ml | Part 1255 |
APPLICATION:
Newcomer Supply Alcian Blue pH 2.5, Barrett’s Esophagus Control Slides are for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin) as well as stromal (mesenchymal) mucin and demonstrates the presence of columnar epithelium with goblet cells and stratified squamous epithelium in esophageal biopsy.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Move slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5 Aqueous. Stain for 30 minutes at room temperature or for 15 minutes in a 37°C water bath.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #3.
- Counterstain in Solution C: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #4
- Dehydrate quickly through two changes of 95% ethyl alcohol and two changes of 100% ethyl alcohol. Clear in three xylene changes, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Blue |
| Stromal (mesenchymal) mucin | Blue |
| Goblet cells | Pale blue |
| Nuclei | Pink-red |
| Cytoplasm | Pale pink |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- A brief dip in Solution A: Acetic Acid 3%, Aqueous from Step #3 can be added before water rinses to remove excess Alcian Blue Solution if needed.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 145-148.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 172-175.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining umbilical cord and positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alcian Blue pH 2.5 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Alcian Blue 1%, pH 2.5 Stain Kit: | Part 9102A/B | Individual Stain Solution | |
| Solution A: | Acetic Acid 3%, Aqueous | 250/500 ml | Part 10017 |
| Solution B: | Alcian Blue Stain 1%, pH 2.5 Aqueous | 250/500 ml | Part 1003 |
| Solution C: | Nuclear Fast Red Stain, Kernechtrot | 250/500 ml | Part 1255 |
APPLICATION:
Newcomer Supply Alcian Blue pH 2.5, Multi-Tissue Control Slides use a combination of tissue sources for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin) as well as stromal (mesenchymal) mucin.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Move slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5 Aqueous. Stain for 30 minutes at room temperature or for 15 minutes in a 37°C water bath.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #3.
- Counterstain in Solution C: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #4.
- Dehydrate quickly through two changes of 95% ethyl alcohol and two changes of 100% ethyl alcohol. Clear in three xylene changes, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Blue |
| Stromal (mesenchymal) mucin | Blue |
| Nuclei | Pink-red |
| Cytoplasm | Pale pink |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- A brief dip in Solution A: Acetic Acid 3%, Aqueous from Step #3 can be added before water rinses to remove excess Alcian Blue Solution if needed.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 145-148.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 172-175.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining umbilical cord.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alcian Blue pH 2.5 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Alcian Blue 1%, pH 2.5 Stain Kit: | Part 9102A/B | Individual Stain Solution | |
| Solution A: | Acetic Acid 3%, Aqueous | 250/500 ml | Part 10017 |
| Solution B: | Alcian Blue Stain 1%, pH 2.5 Aqueous | 250/500 ml | Part 1003 |
| Solution C: | Nuclear Fast Red Stain, Kernechtrot | 250/500 ml | Part 1255 |
APPLICATION:
Newcomer Supply Alcian Blue pH 2.5, Umbilical Cord Control Slides are for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin) as well as stromal (mesenchymal) mucin.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Move slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5 Aqueous. Stain for 30 minutes at room temperature or for 15 minutes in a 37°C water bath.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #3.
- Counterstain in Solution C: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #4
- Dehydrate quickly through two changes of 95% ethyl alcohol and two changes of 100% ethyl alcohol. Clear in three xylene changes, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Blue |
| Stromal (mesenchymal) mucin | Blue |
| Nuclei | Pink-red |
| Cytoplasm | Pale pink |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- A brief dip in Solution A: Acetic Acid 3%, Aqueous from Step #3 can be added before water rinses to remove excess Alcian Blue Solution if needed.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 145-148.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 172-175.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: AFB, Ziehl-Neelsen quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With AFB, Ziehl-Neelsen Stain Kit: | Part 9101A | Individual Stain Solution | |
| Solution A: | Carbol Fuchsin Stain, Ziehl-Neelsen | 250 ml | Part 1030 |
| Solution B: | Acid Alcohol 1% | 250 ml | Part 10011 |
| Solution C: | Light Green SF Yellowish Stain 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
Newcomer Supply Acid Fast Bacteria (AFB), Animal Control Slides are for the positive histochemical staining of acid-fast mycobacteria in tissue sections.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution A: Carbol Fuchsin Stain, Ziehl-Neelsen with filter paper whenever a thick sheen develops on solution surface.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in Solution A: Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature. Keep solution covered.
-
- See Procedure Note #3.
-
- Rinse in running tap water for 2 to 3 minutes.
- Differentiate in Solution B: Acid Alcohol 1% until color no longer runs off the slide and sections are pale pink; 3 to 10 rapid dips.
- Wash in running tap water 3 to 5 minutes; rinse in distilled water.
- Counterstain in Solution C: Light Green SF Yellowish Stain 0.1%, Aqueous; 2-5 dips.
- Rinse with one quick dip in distilled water or proceed directly to Step #10 without a distilled water rinse.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Acid-fast bacilli | Bright red |
| Background | Green |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Sections can remain in Carbol Fuchsin Stain, Ziehl-Neelsen for up to 60 minutes without adverse effect. Additional differentiation may be required in Step #6.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-instructional Text. 5th ed. Chicago: ASCP Press, 2020. 213-215.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.
Easily store your Slides and Tissue Paraffin Blocks in this sturdy and convenient system!
TISSUE PARAFFIN BLOCK STORAGE SYSTEM:
- 8 drawers holds 500 blocks in a box
- 5,000 block storage per case
SLIDE STORAGE SYSTEM:
- 4 drawers hold 2,000 slides in a box
- 20,000 slide storage per case
DIMENSIONS FOR BOTH SLIDE & BLOCK STORAGE BOXES:
- 12″ (W) x 3 ¾”(H) x 11 ½” (L)
TISSUE PARAFFIN BLOCK & SLIDE STORAGE SYSTEM:
- Well organized & detailed recording area on front panel of each box
- Easily identify the slides or blocks contained in the box
- From:/To: stickers available to place over recording area on front panel as well as on each drawer
- Out/In place markers available to keep track of slides/blocks that have been removed
- Easily stackable
- Great pricing!
- Made in the USA
Samples of accessories in each case!
This sturdy, heavy-duty, high capacity metal slide storage cabinet is an ideal solution for the safe, long-term storage of slide specimens. Each cabinet unit holds up to 4,500 slides. Up to 10 units can be secured together on a single base for a total of 45,000 slides per stack!
BUILD YOUR SLIDE FILING SYSTEM!
1. The Base
.jpg)
- Sturdy base can hold up to 10 cabinets
- Available in Tan or Green
2. Slide Cabinet

- 6 Drawers with brass pull knobs per cabinet.
- Suspension design to help prevent accidental drawer pull out.
- Cabinets lock together to protect against movement of cabinet and conserve space.
- Each drawer section accommodates up to 4,500 slides.
- Dimensions: 18 ¾” x 15 ¾” x 5″H
- Available in Tan or Green.
3. Stack Cabinets together to keep storage in one area.
.jpg)
BUILD YOUR SLIDE FILING SYSTEM!
1. The Base
.png)
- Sturdy base to hold 6 drawer slide cabinet.
- Available in brown.
2. Slide Cabinet

-
6 Drawers with black pull knobs per cabinet.
-
Cabinets lock together to protect against movement of cabinet and conserve space.
-
Each cabinet (6 drawers) accommodates up to 5,000 slides.
-
Each drawer comes with a foam stop block to keep slides standing upright when the drawer is not completely full.
-
Dimensions: 18 ¾” x 15 ¾” x 5″H
-
Available in brown.
3. Stack Cabinets on base and together (up to 10 high) to keep storage in one area.

(use: Fite Stain for Nocardia & Leprosy.)
(use: Alcian Blue, pH 2.5.)
SOLUTION:
| Gallon | |
| Saccomanno Collection Fluid | Part 1361B |
| 60 ml vial, 30 ml fill (50/cs) | |
| Saccomanno Collection Fluid Vial | Part 1361D |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Saccomanno Collection Fluid is a ready-to-use cytology pre-fixative and sample preservative, and fluid of choice for collection of sputum specimens. Saccomanno Collection Fluid also provides a variety of applications for collection and transport of cytology specimens, such as:
-
- Fine need aspirates (FNA)
- Urine samples
- Urinary tract washings
- Bronchial washings and brushings
- Bronchoalveolar lavage (BAL)
- Gastrointestinal specimens
Saccomanno Collection Fluid works to partially fix and stabilize cytology specimens, protecting cells from decomposition and autolysis when specimen transport is required or when cytopreparation is delayed. Mucoproteins will remain suspended in the collection fluid and cells can be reclaimed with centrifugation.
METHOD:
Fixation: Add equal volume of Saccomanno Collection Fluid to cytology specimen.
-
-
- For brushes, add enough collection fluid to fully cover.
-
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
FIXATION PROCEDURE:
-
- After cytology specimen is collected and received in the laboratory, add equal volume of Saccomanno Collection Fluid to specimen that will be transported or held for an extended amount of time prior to cytopreparation.
-
- See Procedure Note #1.
-
- Store and/or transport specimen according to suggested temperature specifications for specimen type.
- Proceed with cytology sample fixation and processing method for specific specimen type.
-
- See Procedure Note #2.
-
- After cytology specimen is collected and received in the laboratory, add equal volume of Saccomanno Collection Fluid to specimen that will be transported or held for an extended amount of time prior to cytopreparation.
PROCEDURE NOTES:
-
- Refer to laboratory guidelines for length of time a specific cytology specimen type can be held fresh prior to processing or addition of Saccomanno Collection Fluid.
- Saccomanno Collection Fluid is considered a cytology pre-fixative and not a substitute for final specimen fixation in appropriate strength alcohol.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-Instructional Text. 4th ed. Chicago: ASCP Press, 2015. 318-321.
- Kini, Sudha R. Color Atlas of Pulmonary Cytopathology. New York: Springer, 2002. 7-21.
- Koss, Leopold G., and Myron Melamed, eds. Koss’ Diagnostic Cytology and Its Histopathologic Bases. Fifth ed. Philadelphia: Lippincott Williams & Wilkins, 2006. 1570-1594.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 1 Liter | 1 Gallon | |
| Zenker Fixative, Modified, Zinc Chloride | Part 1461A | Part 1461B |
Additionally Needed:
| Acetic Acid, Glacial, ACS | Part 10010 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Zenker Fixative, Modified, Zinc Chloride is a safe mercury free alternative to the original Zenker formulation. Zenker Fixative, Modified, Zinc Chloride is a specialty fixative that provides crisp nuclear detail and is frequently used for bone marrow clots and biopsies.
The acidic nature of Zenker Fixative, Modified, Zinc Chloride Working Solution is generally enough to sufficiently decalcify bone marrow cores without additional decalcification steps.
METHOD:
Fixation: Recommended fixation time is a minimum of 2 to 4 hours, depending upon tissue size and type.
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
FIXATION PROCEDURE:
-
- Zenker Fixative, Modified, Zinc Chloride Working Solution:
-
- Zenker Fixative, Modified, Zinc Chloride 38 ml
- Acetic Acid, Glacial, ACS 2 ml
-
- Combine and mix solutions directly before use. Fix tissue in this fresh working solution for 2 to 4 hours depending upon tissue size and type.
- Hold tissue specimens in Zenker Fixative, Modified, Zinc Chloride Working Solution until ready to process or a maximum of 24 hours.
-
- See Procedure Note #1.
-
- Wash fixed tissue in running tap water for minimum of 10 minutes to remove residual zinc chloride.
- Place on tissue processor in Formalin 10%, Phosphate Buffered (Part 1090) fixation step.
- Post-fixation applications of Zenker Fixative, Modified, Zinc Chloride include the use of the working fixative as a mordant in phosphotungstic acid hematoxylin (PTAH) staining procedures. Refer to PTAH stain protocol for additional information.
- Zenker Fixative, Modified, Zinc Chloride Working Solution:
PROCEDURE NOTES:
-
- After maximum fixation time of 24 hours, rinse tissue in running tap water for a minimum of 10 minutes; transfer Zenker fixed wet tissue to 70% Ethyl Alcohol (Part 10844) or Formalin 10%, Phosphate Buffered for long-term storage.
- Zenker Fixative, Modified, Zinc Chloride is not recommended for preservation of red blood cells.
- Due to its corrosive nature, do not discard Zenker Fixative, Modified, Zinc Chloride solutions down the drain.
- Neutralize Zenker Fixative, Modified, Zinc Chloride with sodium carbonate or sodium bicarbonate to precipitate zinc at pH 7.0-8.0.
-
-
- Approximately 100 grams of sodium bicarbonate will neutralize/precipitate zinc from 1 liter of Zenker Fixative, Modified, Zinc Chloride.
-
-
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 16-17, 20-21, 207-208.
- Dapson, Janet Crookham, and Richard Dapson. Hazardous Materials in the Histopathology Laboratory: Regulations, Risks, Handling, and Disposal. 4th ed. Battle Creek, MI: Anatech, 2005. 148, 279.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 49.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 1 Liter | 6 X 1 Liter | 1 Gallon | |
| B-5 Fixative Modified, Zinc Chloride | Part 1015A | Part 1015A | Part 1015C |
Additionally Needed:
| Formaldehyde 37-40%, ACS | Part 1089 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply B-5 Fixative Modified, Zinc Chloride is a safe mercury free alternative to the classic B-5 fixative. It is the fixative of choice for bone marrow, lymph nodes, spleen and other hematopoietic tissues, and provides clear nuclear detail while preserving immunohistochemical (IHC) staining.
METHOD:
Fixation Recommendations:
- Bone Marrow Clot: Minimum of 2 hours.
- Bone Marrow Biopsy: Minimum of 3 hours.
- Lymph Nodes and Small Biopsies: Minimum of 4 hours.
- Small nodes (5 mm or less) should be halved.
- Larger nodes, dissected so no piece is thicker than 3 mm.
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
FIXATION PROCEDURE:
-
- B-5 Fixative Modified, Zinc Chloride Working Solution:
-
- B-5 Fixative Modified, Zinc Chloride 50 ml
- Formaldehyde 37-40%, ACS 5 ml
-
- Combine solutions directly before use. Fix tissue in fresh 10:1 working solution a minimum of 2 to 4 hours.
- Hold tissue specimens in B-5 Fixative Modified, Zinc Chloride Working Solution until ready to process or a maximum of 72 hours.
-
- See Procedure Note #1.
-
- Wash fixed tissue in running tap water to remove residual zinc chloride.
- Place on tissue processor in Formalin 10%, Phosphate Buffered (Part 1090) fixation step.
- B-5 Fixative Modified, Zinc Chloride Working Solution:
PROCEDURE NOTES:
-
- After maximum fixation of 72 hours, transfer B-5 fixed wet tissue to 70% Ethyl Alcohol (Part 10844) for long-term storage.
- Nitric acid is not recommended as a decalcification agent following fixation in B-5 Fixative Modified, Zinc Chloride.
- Neutralize B-5 Fixative Modified, Zinc Chloride with sodium carbonate or sodium bicarbonate to precipitate zinc at pH 7.0-8.0.
-
- Approximately 100 grams of sodium bicarbonate will neutralize/precipitate zinc from 1 liter of B-5 Fixative Modified, Zinc Chloride.
-
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 69.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-Instructional Text. 4th ed. Chicago: ASCP Press, 2015. 19-20.
- Dapson, Janet Crookham, and Richard Dapson. Hazardous Materials in the Histopathology Laboratory: Regulations, Risks, Handling, and Disposal. 4th ed. Battle Creek, MI: Anatech, 2005. 148-149, 279.
- Modifications developed by Newcomer Supply Laboratory.
SOLUTION:
| 500 ml | 1 Liter | 1 Gallon | |
| Wright Stain, Buffered | Part 1422A | Part 1422B | Part 1422C |
Additionally Needed:
| Alcohol, Methanol Anhydrous, ACS | Part 12236 |
| Wright Stain Buffer, pH 6.8 | Part 1430 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Wright Stain, Buffered for Smears provides a quick staining technique for differential staining of cell types in peripheral blood smears as well as bone marrow smears/films.
METHOD:
Technique: Coplin jar or flat staining rack method
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
PRESTAINING PREPARATION:
-
- Prepare within an accepted time frame, a well-made blood smear or bone marrow smear/film per your laboratories protocol, with a focus on uniform cell distribution.
- Allow slides to thoroughly air-dry prior to staining.
- Filter Wright Stain, Buffered prior to use with quality filter paper.
-
- For flat staining rack method, filter sufficient stain to allow 1 ml of stain per slide.
-
- Prepare 25% Aqueous Methanol Rinse; combine and mix well.
-
- Distilled Water 30 ml or 3 ml
- Methanol (Part 12236) 10 ml or 1 ml
-
STAINING PROCEDURE:
-
- Coplin Jar Method: See Procedure Notes #1 and #2.
-
- Fix smears in Methanol for 15 seconds.
- Stain in filtered Wright Stain, Buffered for 1-2 minutes.
- Place directly into Wright Stain Buffer, pH 6.8 (Part 1430), for 1-4 minutes. Do Not Agitate!
- Dip quickly in 25% Aqueous Methanol Rinse (Step #4).
- Rinse in distilled water.
- Air-dry slides in a vertical position; examine microscopically.
- If coverslip is preferred, allow slides to air-dry and coverslip with compatible mounting medium.
-
- Flat Staining Rack Method: See Procedure Notes #1 and #2.
-
- Place slides on flat staining rack suspended over sink.
- Fix by flooding slide with Methanol for 15 seconds.
- Drain off Methanol.
- Flood each slide with 1 ml of filtered Wright Stain, Buffered for 1 minute.
- Retain Wright Stain, Buffered on slides.
- Directly add 2 ml of Wright Stain Buffer, pH 6.8 to each slide; agitate gently to mix with retained Wright Stain.
- Stain for an additional 3 minutes.
- Flood smears with 25% Aqueous Methanol Rinse (Step #4) for 1 second.
- Rinse in distilled water.
- Air-dry slides in a vertical position; examine microscopically.
- If coverslip is preferred, allow slides to air-dry and coverslip with compatible mounting medium.
-
- Coplin Jar Method: See Procedure Notes #1 and #2.
RESULTS:
| Erythrocytes | Pink |
| Granules – Purple | |
| Eosinophils | Granules – Pink |
| White blood cells | Chromatin – Purple |
| Lymphocytes | Cytoplasm – Blue |
| Cytoplasm – Blue | |
| Bacteria | Deep Blue |
PROCEDURE NOTES:
-
- Timings provided are suggested ranges. Optimal times will depend upon staining intensity preference.
- Smears containing primarily normal cell populations require minimum staining time; immature cells and bone marrow smears/films may require longer staining time.
- The color range of stained cells may vary depending on buffer pH and pH of rinse water.
-
- Alkalinity is indicated by red blood cells being blue-grey and white blood cells only blue.
- Acidity is indicated by red blood cells being bright red or pink and lack of proper staining in white blood cells.
- If necessary, adjust buffer pH accordingly to 6.8 +/ – 0.2.
-
REFERENCES:
-
- Lillie, R. D., and Harold Fullmer. Histopathologic Technic and Practical Histochemistry. 4th ed. New York: McGraw-Hill, 1976. 747-748.
- McPherson, Richard and Matthew Pincus. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia: Elsevier Saunders, 2011. 522-532.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 154-155.
- Modifications developed by Newcomer Supply Laboratory.
Armadillo spleen with mycobacterium leprae the causative organism for leprosy.
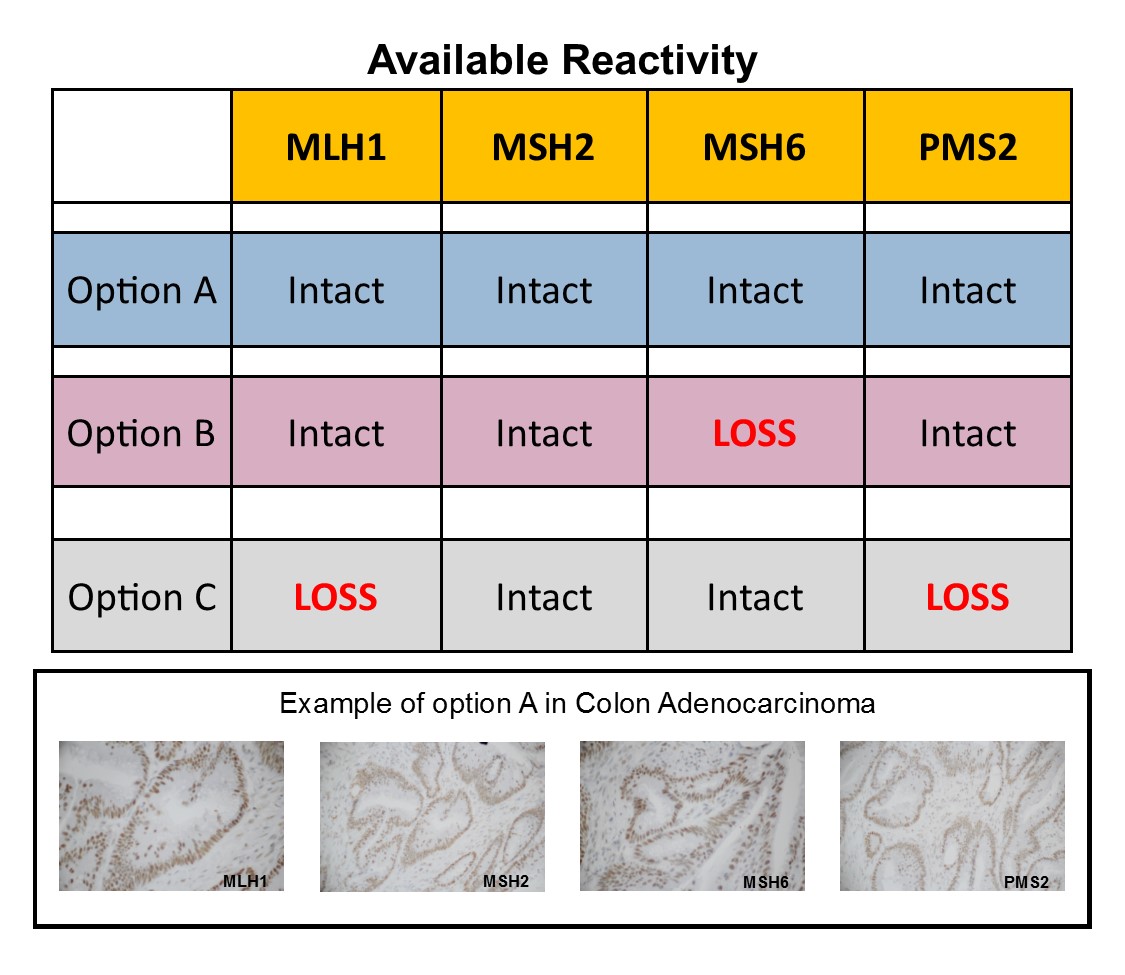
Colon carcinoma that expresses positive reactivity in each of the MMR panel of four markers; MLH1, MSH2, MSH6 and PMS2. Mismatch Repair testing is useful in screening for colorectal carcinoma (CRC), Microsatellite Instability (MSI) and Lynch Syndrome (LS).