PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: BCL2 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply BCL2 (B-cell lymphoma 2) Control Slides are for the positive immunohistochemical staining of BCL2. Frequently used to distinguish positive staining follicular lymphoma from negative reacting follicular hyperplasia.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply BCL2 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| BCL2 positive expression | Brown cytoplasmic staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque BCL2 (124) is the concentrated primary antibody used. Dilute primary antibody to 1/25 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque BCL2 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Gram positive staining rat lung and gram negative staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Brown-Brenn quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Gram, Brown-Brenn, Stain Kit: | Part 9123A | Individual Stain Solution | |
| Solution A: | Crystal Violet-Oxalate Stain, Alcoholic | 250 ml | Part 10422 |
| Solution B: | Iodine, Gram, Aqueous | 250 ml | Part 1140 |
| Solution C: | Acetone-Alcohol 1:1 | 250 ml | Part 10016 |
| Solution D: | Basic Fuchsin Stain 0.25%, Aqueous | 250 ml | Part 1011 |
| Solution E: | Tartrazine Stain 0.25%. Acetic Aqueous | 250 ml | Part 14016 |
APPLICATION:
Newcomer Supply Gram, Multi-Tissue, Artificial Control Slides are for the positive histochemical staining of gram positive and gram negative bacteria in separate tissue sections. Escherichia coli and Staphylococcus aureus are used to produce the positive controls.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution A: Crystal Violet-Oxalate Stain, Alcoholic.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in freshly filtered Solution A: Crystal Violet-Oxalate Stain, Alcoholic (Step #2) for 1 minute.
- Rinse well in distilled water.
- Mordant in Solution B: Iodine, Gram, Aqueous for 1 minute.
- Rinse well in distilled water, removing excess iodine.
- Decolorize in Solution C: Acetone-Alcohol 1:1 until blue stops running; 7-10 dips.
- Rinse well in distilled water.
- Place in Solution D: Basic Fuchsin Stain 0.25%, Aqueous for 90 seconds.
- Rinse well in distilled water.
- Dip once in Solution C: Acetone-Alcohol 1:1.
- Counterstain in Solution E: Tartrazine Stain 0.25%, Acetic Aqueous for 5-15 seconds.
- Rinse well in distilled water.
- Dehydrate in two changes of 100% ethyl alcohol, 5 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
- Do not use 95% alcohol in the dehydration step.
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Gram negative bacteria | Red |
| Gram positive bacteria | Blue/violet |
| Background tissue | Yellow |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 312-313.
- Brown, J.H., and L. Brenn. “A Method for the Differential Staining of Gram Positive and Gram Negative Bacteria in Tissue Sections”. Bulletin of The Johns Hopkins2 (1931): 69-73.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 188-189.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Brown-Brenn quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Gram, Brown-Brenn Stain Kit: | Part 9123A | Individual Stain Solution | |
| Solution A: | Crystal Violet-Oxalate Stain, Alcoholic | 250 ml | Part 10422 |
| Solution B: | Iodine, Gram, Aqueous | 250 ml | Part 1140 |
| Solution C: | Acetone-Alcohol 1:1 | 250 ml | Part 10016 |
| Solution D: | Basic Fuchsin Stain 0.25%, Aqueous | 250 ml | Part 1011 |
| Solution E: | Tartrazine Stain 0.25%. Acetic Aqueous | 250 ml | Part 14016 |
APPLICATION:
Newcomer Supply Gram Positive & Gram Negative Bacteria, Artificial Control Slides are for the positive histochemical staining of gram positive and gram negative bacteria in a single tissue section. Escherichia coli and Staphylococcus aureus purchased from Remel Microbiology Products are used to produce the positive controls.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution A: Crystal Violet-Oxalate Stain, Alcoholic.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in freshly filtered Solution A: Crystal Violet-Oxalate Stain, Alcoholic (Step #2) for 1 minute.
- Rinse well in distilled water.
- Mordant in Solution B: Iodine, Gram, Aqueous for 1 minute.
- Rinse well in distilled water, removing excess iodine.
- Decolorize in Solution C: Acetone-Alcohol 1:1 until blue stops running; 7-10 dips.
- Rinse well in distilled water.
- Place in Solution D: Basic Fuchsin Stain 0.25%, Aqueous for 90 seconds.
- Rinse well in distilled water.
- Dip once in Solution C: Acetone-Alcohol 1:1.
- Counterstain in Solution E: Tartrazine Stain 0.25%, Acetic Aqueous for 5-15 seconds.
- Rinse well in distilled water.
- Dehydrate in two changes of 100% ethyl alcohol, 5 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
- Do not use 95% alcohol in the dehydration step.
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Gram positive bacteria | Blue/violet |
| Gram negative bacteria | Red |
| Background tissue | Yellow |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 312-313.
- Brown, J.H., and L. Brenn. “A Method for the Differential Staining of Gram Positive and Gram Negative Bacteria in Tissue Sections”.Bulletin of The Johns Hopkins2 (1931): 69-73.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 188-189.
- Modifications developed by Newcomer Supply Laboratory.
FEATURES OF THE BLOCK STORAGE SYSTEM, PLASTIC 6 DRAWER UNIT:
-
- Made of ABS
- Six segmented, impact resistant drawers
- Seven segmented rows per drawer. Each row includes a sponge block to support blocks in upright position.
- Identification writing area on each drawer
- Stackable cabinets have locking features to each other
- Each 6 drawer unit accommodates up to 2,100 FFPE tissue block cassettes
- Cabinet Dimension: 17 3/8″L x 9 1/2″W x 15 1/4″H
- Interfaces with other popular plastic block storage systems!
Block filing cabinet drawer
Locking pegs at each corner for stacking multiple units on one another
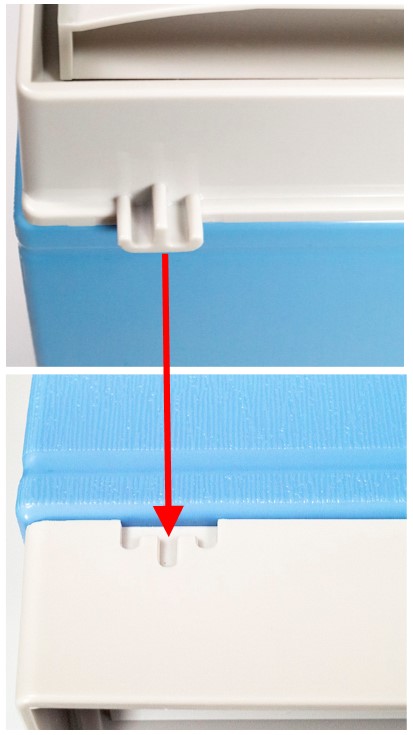
Filing cabinets stack securely together
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori Prussian Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Iron, Gomori Prussian Blue Stain Kit: | Part 9136A/B | Individual Stain Solution | |
| Solution A: | Hydrochloric Acid 20%, Aqueous | 125/250 ml | Part 12087 |
| Solution B: | Potassium Ferrocyanide 10%, Aqueous | 125/250 ml | Part 13392 |
| Solution C: | Nuclear Fast Red Stain, Kernechtrot | 250/500 ml | Part 1255 |
APPLICATION:
Newcomer Supply Iron, Animal Control Slides are for the positive histochemical staining of ferric iron deposits in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol
- To avoid the possibility of residual background iron staining, acid clean glassware is recommended in the staining procedure.
- See Procedure Note #1.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #2 and #3.
- Prepare fresh Ferrocyanide Working Solution directly before use; combine and mix well.
- Solution A: Hydrochloric Acid 20%, Aqueous 20 ml
- Solution B: Potassium Ferrocyanide 10%, Aqueous 20 ml
- Place slides in fresh Ferrocyanide Working Solution for 20 minutes.
- Rinse in three changes of tap water; rinse in distilled water.
- Place in Solution C: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #4.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Ferric iron deposits | Bright blue |
| Nuclei | Red |
| Cytoplasm | Pink |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 179-184.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 217-218.
- Modifications developed by Newcomer Supply Laboratory.
The Activate™ Bleach Sprayer Dilution System has been made to meter and dispense 5.25% Sodium Hypochlorite NaOCL at a 10% (9:1) solution. It has been widely recommended by CDC and other nationally recognized authorities.
FEATURES AND BENEFITS OF THE ACTIVATE™ BLEACH SPRAYER DILUTION SYSTEM:
- Automatically mixes water and bleach to make a fresh 10% solution every time the trigger is pulled.
- Prevents bleach from degrading (ALWAYS AT FULL STRENGTH).
- Eliminates safety hazard of mixing bleach by hand!
- Fast contact time for practical disinfecting.
- Sealed system prevents closed cap concerns.
- Non-aerosol coarse spray.
- Easy to install disposable bleach cartridges make approximately one gallon useable solution.
- Sustainable packaging reduces plastic waste and saves in freight costs.
- Ergonomic trigger sprayer is comfortable for high use.
ACTIVATE™ BLEACH SPRAYER DILUTION SYSTEM EFFICACY SUMMARY:
Activate™ 5.25% Institutional Bleach is effective against a broad spectrum of pathogens when used as directed.
| Pathogen | Contact Time |
| HIV-1 (AIDS) | 30 seconds |
| HBV (Hepatitis B) | 30 seconds |
| MRSA (Methicillan-resistant Staphylococcus aureus) | 2 minutes |
| Staphylococcus aureus | 2 minutes |
| Pseudomonas aeruginosa | 2 minutes |
| Salmonella enterica | 2 minutes |
| Streptococcus Pyogenes | 2 minutes |
| VRE (Vancomycin-resistant Enterococcus faecalis) | 2 minutes |
| Acinetobacter baumannii | 2 minutes |
| Clostridium difficile Endospores | 4 minutes |
SPECIFICATIONS OF THE ACTIVATE™ BLEACH SPRAYER DILUTION SYSTEM:
| Active Ingredient: | 5.25% Sodium Hypochlorite |
| Dilution: | 5000ppm |
| EPA Reg. No.: | 75266-1 |
| Personal Protection: | Gloves, eye & clothing protection |
| Ventilation Requirement: | Moderate ventilation required |
| Solubility: | 100% in water |
| Odor when Diluted: | Light Chlorine |
| pH when Diluted: | 9.5 |
| Fire Hazard: | None |
| Explosion Hazard: | None |
| Storage Temperature: | 50° to 72°F |
| Shelf Life: | Degrades; periodically test with chlorine test strips |
| Incompatibilities: | Ammonia, acids, reducing agents, heavy metals |
HOW TO USE THE ACTIVATE BLEACH SPRAYER DILUTION SYSTEM:
Step 1: Fill the bleach sprayer’s empty water bottle with cool tap water, and then insert the sprayer’s tube down into the filled water bottle. Close the bleach sprayer tab to lock the water bottle into place on the sprayer.

Step 2: Remove the threaded plastic cap from the bleach sprayer cartridge. Do not puncture or tamper with the plug that is in the neck of the bleach sprayer cartridge. It must remain intact in order for the bleach sprayer to dilute the bleach at the proper 10% dilution rate.

Step 3: Lock the bleach sprayer’s cartridge onto the other side of the sprayer.
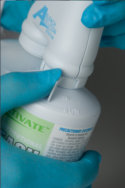
Step 4: Pointing the bleach sprayer away from you into a sink or basin, pump the trigger 20 times to prime the system.
Step 5: The bleach sprayer is now ready to use. Hold the sprayer 6″ to 8″ from the surface, and spray thoroughly until the surface is completely wet.
Step 6: Refill the water bottle when empty.
Step 7: Always re-prime the bleach sprayer when installing a new bleach cartridge. Note that a safety feature ensures the bleach sprayer will not function if either the bleach sprayer’s cartridge or the water bottle is empty. Periodically test the sprayed bleach solution for available chlorine with a high-level chlorine test strip. Always store your bleach system and additional bleach cartridges at room temperature (50º – 72º).

Note: The bleach sprayer mixes the bleach with the water to a 10% ( 1 part bleach to 9 parts water ) solution (minimum 5,000 ppm available chlorine), so the water cartridge will need to be filled 9 times before the bleach cartridge is emptied.
**Always remove gross dirt and debris from surface prior to disinfection.
- Spray Activate™ 6-8″ from surface until surface is thoroughly wet.
- Let stand for 2 minutes. To kill HIV-1 and HBV, allow 30 seconds contact time at room temperature.
- Wipe with a clean cloth or paper towel and allow to air dry.
- For food contact surfaces, rinse with potable water.
INSTRUCTIONS FOR CLEANING PRIOR TO DISINFECTING AGAINST CLOSTRIDIUM DIFFICILE SPORES:
- Personal Protection: Wear appropriate barrier protection such as gloves, gowns, masks or eye covering.
- Cleaning Procedure: Thoroughly clean fecal matter/waste from surfaces/objects before disinfection by application with a clean cloth, mop, and/or sponge saturated with Activate™. Vigorously wipe and/or scrub surfaces/object until all visible soil is removed. Pay special attention to high-touch surfaces. Clean surfaces in patient rooms in an appropriate manner, such as from right to left or left to right on horizontal surfaces, and top to bottom on vertical surfaces, to minimize spreading of the spores. Clean restrooms last. Do not reuse soiled cloths.
- Infectious Materials Disposal: Immediately dispose of materials used in the cleaning process that may contain feces/waste in accordance with local regulations for infectious materials disposal.
- Disinfection Procedure: Spray Activate™ 6-8″ from surface until surface is thoroughly wet; let stand 4 minutes. Wipe with a clean, damp cloth or paper towel, or allow to air dry.
TO PRE-CLEAN INSTRUMENTS PRIOR TO TERMINAL STERLILIZATION:
- Place instruments in a suitable container.
- Spray Activate™ on instruments until all surfaces are thoroughly wet.
- Allow to stand for up to 10 minutes.
- Rinse instruments with water, and follow with an appropriate sterilization/high-level disinfecting process.
- Cleaning of critical and semi-critical devices must be followed by an appropriate terminal sterilization/high-level disinfection process.
NOTE: Use Active™ on stainless steel instruments only.
TO DISINFECT NON-CRITICAL, PRE-CLEANED INSTRUMENTS:
- Instruments first must be thoroughly cleaned to remove gross dirt and debris, rinsed, and dried.
- Place instruments in a suitable container.
- Spray Activate™ on instruments until all surfaces are thoroughly wet.
- Let stand for 2 minutes. To kill HIV-1 and HBV, allow 30 seconds contact time at room temperature. To kill Clostridium difficile spores, let stand for 4 minutes.
- Wipe with a clean, damp cloth or paper towel, and allow to air dry.
This product is not to be used as a terminal sterilant/high-level disinfectant on any surface or instrument that (1) is introduced directly into the human body, either into or in contact with the bloodstream or normally sterile areas of the body, or (2) contacts intact mucous membranes but which does not ordinarily penetrate the blood barrier or otherwise enter normally sterile areas of the body. This product may be used to pre-clean or decontaminate critical or semi-critical medical devices prior to sterilization or high-level disinfection.
Kills HIV and HBV on pre-cleaned environmental surfaces and objects previously soiled with blood/body fluids in healthcare setting or other settings in which there is an expected likelihood of soiling of inanimate surfaces/objects with blood/body fluids, and in which the surfaces/objects likely to be soiled with blood/body fluids can be associated with the potential for transmission of Human Immunodeficiency Virus Type-1 (HIV-1) (associated with AIDS) and Hepatitis B Virus (HBV).
SPECIAL INSTRUCTIONS FOR CLEANING & DECONTAMINATING AGAINST HIV-1 AND HBV ON SURFACES/OBJECTS SOILED WITH BLOOD/BODY FLUIDS:
- When handling items soiled with blood/body fluids, personal protective equipment such as disposable gloves, gowns, masks, and eye coverings must be used.
- Blood/body fluids must be thoroughly cleaned from surfaces/objects prior to disinfecting. Spray Activate™ on surface/object until all surfaces are thoroughly wet, or apply with a clean cloth, mop or sponge saturated with Activate™; let stand for 30 seconds. Wipe with a clean cloth or paper towel, and allow to air dry.
- Blood/body fluids and cleaning materials must be autoclaved and disposed of according to federal, state, and local regulations for infectious waste disposal.
TO CLEAN AND DISINFECT IN A VETERINARY APPLICATION:
- Use to clean and disinfect hard, non-porous surfaces such as feeding and watering equipment, cages, utensils, instruments, kennels, stables, catteries, etc.
- Remove all animals and feed from premises, animal transportation vehicles, crates, etc.
- Remove all litter, droppings, and manure from walls, floors, and surfaces of facilities occupied or traversed by animals.
- Empty all feeding and watering equipment.
- Pre-clean all surfaces with soap or detergent and rinse with water.
- Spray Activate™ on surface/object until all surfaces are saturated, or apply with a clean cloth, mop or sponge saturated with Activate™, let stand for 2 minutes. To kill HIV-1 and HBV, allow 30 seconds at room temperature.
- Rinse all surfaces with potable water.
- Ventilate buildings and other closed spaces. Do not house animals or employ equipment until treated surfaces have been thoroughly rinsed with water and allowed to dry. Thoroughly scrub all treating, feeding, and watering applications with soap or detergent, and rinse with potable water before reuse.
STORAGE AND DISPOSAL OF THE ACTIVATE™ BLEACH:
Do not contaminate food or feed by storage, disposal or cleaning of equipment.
Pesticide Storage: This product degrades with age; test active level of diluted product periodically with high-level chlorine test strips. Store in a cool (50°-72°F), dry area away from direct sunlight and heat to avoid deterioration. In case of a spill, flood area with large quantities of water.
Pesticide Disposal: Products or rinsates that cannot be used should be diluted with water before disposal in a sanitary sewer.
Container Handling: Non-refillable container. Do not re-use or refill this container. Offer for recycling if available or place in trash collection.
.jpg)
VioNexus™ No-Rinse Spray Handwash is an alcohol-based hand sanitizer for protection against bacteria when hands are not visibly soiled. VioNexus encourages proper hand hygiene practice by making it convenient, efficient, and comfortable.
VIONEXUS NO-RINSE SPRAY HANDWASH FEATURES AND BENEFITS:
- Meets CDC Guidelines for Hand Hygiene in Healthcare Settings
- Kills 99.9% of bacteria on skin without running water
- Contains 72% ethanol, killing germs in seconds
- Emollients help prevent dry, irritated skin and leave hands feeling soft
- No water or towels needed, eliminating cross contamination
- Fast drying for quick donning of gloves
VIONEXUS NO-RINSE SPRAY HANDWASH LIST OF USES:
- Animal care facilities
- Break rooms
- Community health centers
- Correctional facilities
- Dental offices
- Dialysis clinics
- Dining areas
- Donning rooms
- Emergency medical settings
- Employee work stations
- Entrances and exits
- Extended care
- General practices
- High-traffic areas
- Hospitals
- Isolation areas
- Laboratories
- Laundry rooms
- Long-term care
- Meeting rooms
- Military bases
- Neonatal units
- Nursing homes
- Operating rooms
- Ophthalmic and optometric facilities
- Orthodontist offices
- Outpatient surgical centers
- Reception desks
- Schools
- Surgical centers
- Transaction counters
- Waiting rooms
PRODUCT SPECIFICATIONS:
Tissue: Positive staining kidney and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: GATA3 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply GATA3 (Guanine-Adenine-Thymine-Adenine binding protein 3) Control Slides are for the positive immunohistochemical staining of GATA3, a transcription factor that plays a role in directing cell proliferation and development, with expression primarily in breast and urothelial carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply GATA3 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| GATA3 positive expression | Brown nuclear staining |
| Adipose | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque GATA3 (L50-823) is the concentrated primary antibody used. Dilute primary antibody to 1/200 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque GATA3 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining kidney and negative staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: WT1 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Wilms’ Tumor (WT1) Control Slides are for the positive immunohistochemical staining of WT1, which plays an important role in cell growth and differentiation and is expressed in kidney, malignant mesothelioma, ovarian carcinoma, gonadoblastoma, nephroblastoma (Wilms’ tumor) and acute myeloid leukemia.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply WT1 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| WT1 positive expression | Brown nuclear staining |
| Lung | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque WT1 (6F-H2) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque WT1 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Vimentin quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Vimentin Control Slides are for the positive immunohistochemical staining of Vimentin, a ubiquitous intermediate filament found primarily in a wide variety of mesenchymal cells. Vimentin is also commonly used to assess the effects of over-fixation and immunoreaction.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Vimentin primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Vimentin positive expression | Brown cytoplasmic staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Vimentin (V9) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Vimentin Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat organ and negative staining human myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Toxoplasma quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Toxoplasma, Animal Control Slides are for the positive immunohistochemical staining of Toxoplasma, a crescent shaped sporozoan found as an intracellular parasite in various tissues.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Toxoplasma gondii primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Toxoplasma positive expression | Golden brown/brown organisms |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Bio SB Toxoplasma gondii Polyclonal is the primary antibody used along with Cell Marque detection and ancillary reagents.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bio SB Toxoplasma gondii Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining thyroid and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: TTF-1 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Thyroid Transcription Factor (TTF-1) Control Slides are for the positive immunohistochemical staining of TTF-1, selectively expressed in lung and thyroid, and aids in the classification of lung and thyroid tumors.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply TTF-1 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Link. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Label. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| TTF-1 positive expression | Brown nuclear staining |
| Myometrium | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Agilent TTF-1 (8G7G3/1) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Agilent LSAB2 (K0675) Visualization Kit provides the Link and Label solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Agilent TTF-1 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Agilent LSAB2 Visualization Kit datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD3, T-Cell quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD3, T-Cell Control Slides are for the positive immunohistochemical staining of T-Cells. CD3 is considered to be a pan-T-cell marker, and widely used in detection of T-cell malignancies, both immature and mature.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD3 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| T-Cell positive expression | Brown membrane staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD3 (MRQ-39) is the concentrated primary antibody used. Dilute primary antibody to 1/250 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD3 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining pancreas and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Synaptophysin quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Synaptophysin Control Slides are for the positive immunohistochemical staining of Synaptophysin, expressed in normal neuroendocrine cells and neoplasms as well as in brain neurons, spinal cord, retina, Paneth cells in the gastrointestinal tract, and gastric parietal cells. It is considered a broad-range marker of neural and neuroendocrine differentiation.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Synaptophysin primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Synaptophysin positive expression | Brown cytoplasmic staining |
| Kidney | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Synaptophysin (polyclonal 336A) is the concentrated primary antibody used. Dilute primary antibody to 1/300 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Synaptophysin Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung and negative staining human lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Spirochete quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Spirochete Treponema, Artificial Control Slides are for the positive immunohistochemical staining of spirochetes, the causative agent of a variety of diseases such as; syphilis, bejel, pinta, yaws and lyme.
Treponema hyodysenteriae purchased from American Type Culture Collection is used to produce the positive control tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Treponema pallidum primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Spirochete positive expression | Brown |
| Negative lung | Negative for spirochetes |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare Treponema pallidum Polyclonal is the primary antibody used along with Cell Marque detection and ancillary reagents.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Biocare Treponema pallidum Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining organ and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: SOX-10 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply SOX-10 (SRY-Box 10) Control Slides are for the positive immunohistochemical staining of the SOX-10 protein, expressed in melanocytes, melanomas, breast carcinomas, gliomas and schwannomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply SOX-10 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| SOX-10 positive expression | Brown nuclear staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30% Aqueous Solution (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque SOX-10 (polyclonal 383A) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque SOX-10 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining melanoma and negative staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: S-100 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply S-100 Control Slides are for the positive immunohistochemical staining of S-100, a ubiquitous protein expressed in a number of cells. Its demonstration is of great value in the identification of several neoplasms, particularly melanomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply S-100 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| S-100 positive expression | Brown cytoplasmic & nuclear staining |
| Lung | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque S-100 (4C4.9) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque S-100 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining prostate and negative staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PSA quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Prostate Specific Antigen (PSA) Control Slides are for the positive immunohistochemical staining of PSA, present in prostate tissue and expressed in the vast majority of prostate carcinomas. It recognizes primary and metastatic prostatic neoplasms and rarely in tumors of non-prostatic origin.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply PSA primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| PSA positive expression | Brown cytoplasmic staining |
| Lung | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque PSA (ER-PR8) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque PSA Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: CK5/14 + p63 + P504S positive staining prostate and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CK5/14 + p63 + P504S quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for six months from date of receipt. Revalidate after six months to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CK5/14 + p63 + P504S Control Slides provide a useful combination of markers helpful in diagnosing prostatic intraepithelial neoplasia (PIN). For ease of screening, CK5/14 + p63 + P504S positive staining is in a single piece of prostate tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CK5/14 + p63 + P504S multiplex primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply MACH 2 Double Stain 2 Polymer. Incubate for 30 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of Vulcan Fast Red Chromogen.
- Tap off excess buffer; apply Vulcan Fast Red. Incubate for 10 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| P504S positive expression | Red granular cytoplasmic staining |
| CK5/14 positive expression | Brown cytoplasmic staining |
| p63 positive expression | Brown nuclear staining |
| Myometrium | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare CK5/14 + p63 + P504S (PPM 225 DS) is the pre-diluted multiplex primary antibody used.
- Biocare MACH 2 Double Stain 2 Polymer Detection Kit (MRCT525) provides the polymer solution used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- Biocare Vulcan Fast Red Chromogen Kit 2 (FR805) is the second chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare CK5/14 + p63 + P504S datasheet.
- Biocare MACH 2 Double Stain 2 Polymer Detection Kit datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Biocare Vulcan Fast Red Chromogen Kit 2 datasheet
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: P504S and CK5/14 positive staining prostate, p63 positive staining prostate and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CK5/14 + p63 + P504S quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for six months from date of receipt. Revalidate after six months to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CK5/14 + p63 + P504S, Multi-Tissue Control Slides provide a useful combination of markers helpful in diagnosing prostatic intraepithelial neoplasia (PIN).
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CK5/14 + p63 + P504S multiplex primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply MACH 2 Double Stain 2 Polymer. Incubate for 30 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of Vulcan Fast Red Chromogen.
- Tap off excess buffer; apply Vulcan Fast Red. Incubate for 10 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill l (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| P504S positive expression | Red granular cytoplasmic staining |
| CK5/14 positive expression | Brown cytoplasmic staining |
| p63 positive expression | Brown nuclear staining |
| Myometrium | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare CK5/14 + p63 + P504S (PPM 225DS) is the pre-diluted multiplex primary antibody used.
- Biocare MACH 2 Double Stain 2 Polymer Detection Kit (MRCT525) provides the polymer solution used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- Biocare Vulcan Fast Red Chromogen Kit 2 (FR805) is the second chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare CK5/14 + p63 + P504S datasheet.
- Biocare MACH 2 Double Stain 2 Polymer Detection Kit datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Biocare Vulcan Fast Red Chromogen Kit 2 datasheet
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining benign prostate and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: p63 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply p63 Control Slides are for the positive immunohistochemical staining of p63, present in normal prostate basal cells and negative in malignant prostate gland tumors. The p63 protein is expressed in the majority of squamous cell carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply p63 primary antibody. Incubate at room temperature for 20 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP-Polymer solution. Incubate for 20 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| p63 positive expression | Brown nuclear staining |
| Myometrium | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare p63 (4A4) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Biocare Van Gogh Yellow Diluent (PD902).
- Biocare Mach 2™ Universal HRP-Polymer Detection (M2U522) is the HRP-Polymer solution used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare p63 Antibody datasheet.
- Biocare Van Gogh Yellow Diluent datasheet.
- Biocare Mach 2™ Universal HRP-Polymer Detection datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining colon and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: p53 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply p53 Control Slides are for the positive immunohistochemical staining of p53, shown to be a prognostic indicator in colorectal, breast, lung and urothelial carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply p53 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| p53 positive expression | Brown nuclear staining |
| Adipose | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque p53 (DO7) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque p53 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining lung and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: p40 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply p40 Control Slides are for the positive immunohistochemical staining of p40, highly specific for squamous and basal cells and selectively expressed in lung squamous cell carcinoma.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply p40 primary antibody. Incubate at room temperature for 20 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP-Polymer solution. Incubate for 20 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| p40 positive expression | Brown nuclear staining |
| Kidney | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare p40 (BC28) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Biocare Van Gogh Yellow Diluent (PD902).
- Biocare Mach 2™ Universal HRP-Polymer Detection (M2U522) is the HRP-Polymer solution used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare p40 Antibody datasheet.
- Biocare Van Gogh Yellow Diluent datasheet.
- Biocare Mach 2™ Universal HRP-Polymer Detection datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining breast and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: p16 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply p16 Control Slides are for the positive immunohistochemical staining of p16, a tumor suppressor protein that plays an important role in cell cycle regulation and acts as a tumor suppressor implicated in the prevention of cancers. p16 can serve as a surrogate marker for high risk HPV in cases of cervical, head and neck and a variety of HPV related carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply p16 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| p16 positive expression | Brown nuclear & cytoplasmic staining |
| Adipose | Negative |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- BD Pharmingen p16 (G175-405) is the concentrated primary antibody used. Dilute primary antibody to 1/20 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- BD Pharmingen p16 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining pancreas and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: NSE quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Neuron-Specific Enolase (NSE) Control Slides are for the positive immunohistochemical staining of NSE, expressed in both normal and neoplastic cells of neuronal and neuroendocrine origin, and can be used for identification of peripheral nerves, neural and neuroendocrine tumors.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Neuron-Specific Enolase primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Link. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Label. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| NSE positive expression | Brown cytoplasmic staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Agilent Neuron-Specific Enolase (BBS/NC/VI-H14) is the primary antibody used. Dilute primary antibody to a 1/3 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Agilent LSAB2 (K0675) Visualization Kit provides the Link and Label solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Agilent Neuron-Specific Enolase (NSE) Antibody datasheet
- Cell Marque Emerald: Antibody Diluent datasheet.
- Agilent LSAB2 Visualization Kit datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining normal colon.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: MLH1, MSH2, MSH6, PMS2 quality control stained slides included.
Reactivity: Guaranteed product specific reactivity for six months from date of receipt. Revalidate after six months to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Mismatch Repair (MMR) Positive Control Slides provide a single tissue source that expresses positive reactivity in each of the MMR panel of four markers; MLH1, MSH2, MSH6 and PMS2. Mismatch Repair testing is useful in screening for colorectal carcinoma (CRC), Microsatellite Instability (MSI) and Lynch Syndrome (LS).
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply MLH1, MSH2, MSH6 and PMS2 primary antibodies. Apply each antibody to an individual slide and tissue section. Incubate each at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP-Polymer solution. Incubate for 20 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in two changes of buffer.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| MLH1 positive expression | Brown nuclear staining |
| MSH2 positive expression | Brown nuclear staining |
| MSH6 positive expression | Brown nuclear staining |
| PMS2 positive expression | Brown nuclear staining |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare MLH1 (G168-15) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Biocare Van Gogh Yellow Diluent (PD902).
- Biocare MSH2 (FE11) is the concentrated primary antibody used. Dilute primary antibody to 1/400 working dilution with Biocare Renoir Red Diluent (PD904).
- Biocare MSH6 (BC/44) is the concentrated primary antibody used. Dilute primary antibody to 1/250 working dilution with Biocare Van Gogh Yellow Diluent.
- Biocare PMS2 (A16-4) is the concentrated primary antibody used. Dilute primary antibody to 1/200 working dilution with Biocare Renoir Red Diluent.
- Biocare Mach 2™ Universal HRP-Polymer Detection (M2U522) is the HRP-Polymer solution used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare MLH1, MSH2, MSH6 and PMS2 Antibody datasheets.
- Biocare Van Gogh Yellow Diluent datasheet.
- Biocare Renoir Red Diluent datasheet.
- Biocare Mach 2™ Universal HRP-Polymer Detection datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining melanoma and negative staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Melan-A (MART-1) quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Melan-A (MART-1) Control Slides are for the positive immunohistochemical staining of Melan-A, expressed in melanocytes of normal skin, retina, nevi, and in the vast majority of melanomas, and is useful in establishing the diagnosis of metastatic melanomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply MART-1 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Melan-A positive expression | Brown cytoplasmic staining |
| Lung | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque MART-1 (A103) is the concentrated primary antibody used. Dilute primary antibody to a 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque MART-1 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD45 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD45, Leucocyte Common Antigen (LCA) Control Slides are for the positive immunohistochemical staining of CD45, routinely used in the differential diagnosis of undifferentiated neoplasms, whenever malignant lymphoma is suspected. A positive result is highly indicative of lymphoid or myeloid origin.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD45 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD45, LCA positive expression | Brown membrane staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD45 (2B11 & PD7/26) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD45 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Lambda quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Lambda Control Slides are for the positive immunohistochemical staining of surface and hidden determinants of lambda light chain on normal and neoplastic B-cells and expressed in B-cell follicles of human lymphoid tissue. Lambda is an important marker to determine monoclonality of B lymphocyte neoplasms.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Lambda primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Link. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Label. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Lambda positive expression | Brown cytoplasmic staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Agilent Lambda Light Chains (polyclonal A0193) is the concentrated primary antibody used. Dilute primary antibody to a 1/1000 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Agilent LSAB2 (K0675) Visualization Kit provides the Link and Label solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Agilent Lambda Light Chains Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Agilent LSAB2 Visualization Kit datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Ki-67 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Ki-67 Control Slides are for the positive immunohistochemical staining of Ki-67, a marker of proliferating cell populations and useful as a prognostic indicator in a number of neoplasms.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Ki-67 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Ki-67 positive expression | Brown nuclear staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Ki-67 (SP6) is the concentrated primary antibody used. Dilute primary antibody to 1/200 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Ki-67 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Kappa quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Kappa Control Slides are for the positive immunohistochemical staining of surface kappa light chain on normal and neoplastic B-cells. Kappa is an important marker to determine monoclonality of B lymphocyte neoplasms.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Kappa primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Kappa positive expression | Brown cytoplasmic staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Kappa (L1C1) is the primary antibody used. Dilute primary antibody to a 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Kappa Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining melanoma and negative staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides
Quality Control Stain: HMB-45 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply HMB-45 (Human Melanoma Black-45) Control Slides are for the positive immunohistochemical staining of HMB-45, expressed in fetal and neonatal melanocytes, junctional and blue nevus cells, and malignant melanoma.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply HMB-45 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| HMB-45 positive expression | Brown cytoplasmic staining |
| Lung | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque HMB-45 (HMB-45) is the concentrated primary antibody used. Dilute primary antibody to 1/150 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque HMB-45 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Hepatitis B Surface Antigen quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Hepatitis B Surface Antigen (HBsAG) Control Slides are for the positive cytoplasmic immunohistochemical staining of Hepatitis B Surface Antigen, a protein present on the surface of the hepatitis B virus (HBV).
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Hepatitis B Surface Antigen primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| HBsAG positive expression | Brown cytoplasmic staining |
| Kidney | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Hepatitis B Surface Antigen (S1-210) is the concentrated primary antibody used. Dilute primary antibody to 1/800 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Hepatitis B Surface Antigen Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining glioblastoma and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Glial Fibrillary Acidic Protein quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Glial Fibrillary Acidic Protein (GFAP) Control Slides are for the positive immunohistochemical staining of GFAP, expressed in astrocytes, Schwann cells, satellite cells, and enteric glial cells. This marker is widely used to differentiate neoplasms of astrocytic origin from other central nervous system neoplasms.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Glial Fibrillary Acidic Protein primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| GFAP positive expression | Brown cytoplasmic staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Glial Fibrillary Acidic Protein (EP672Y) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Glial Fibrillary Acidic Protein Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: E-cadherin quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply E-cadherin Control Slides are for the positive immunohistochemical staining of E-cadherin, expressed in epithelial lineage and glandular epithelium cells as well as adenocarcinoma of the lung, gastrointestinal tract, and ovary.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply E-cadherin primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Mayer Modified (Part 1202) for 2-5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| E-cadherin positive expression | Brown membrane staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque E-cadherin (EP700Y) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Cell Marque E-cadherin Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining gastrointestinal tract and negative staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Helicobacter quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Helicobacter Control Slides are for the positive immunohistochemical staining of spiral shaped Helicobacter bacterium, strongly associated with inflammation of the stomach and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Helicobacter Pylori primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Helicobacter sp. positive expression | Golden to dark brown bacteria |
| Lung | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Helicobacter pylori Polyclonal is the primary antibody used along with Cell Marque detection and ancillary reagents.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Cell Marque Helicobacter pylori Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal gastrointestinal tract and negative staining human lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Helicobacter quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Helicobacter, Animal Control Slides are for the positive immunohistochemical staining of spiral shaped Helicobacter bacterium, strongly associated with inflammation of the stomach and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Helicobacter Pylori primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Helicobacter sp. positive expression | Golden to dark brown bacteria |
| Lung | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Helicobacter pylori Polyclonal is the primary antibody used along with Cell Marque detection and ancillary reagents.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Cell Marque Helicobacter pylori Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung and negative staining human lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Helicobacter quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Helicobacter, Artificial Control Slides are for the positive immunohistochemical staining of spiral shaped Helicobacter bacterium, strongly associated with inflammation of the stomach and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
Helicobacter pylori purchased from American Type Culture Collection is used to produce the positive control tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Helicobacter Pylori primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Helicobacter positive expression | Golden to dark brown bacteria |
| Lung | Negative |
| Background staining possible | |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Helicobacter pylori Polyclonal is the primary antibody used along with Cell Marque detection and ancillary
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Cell Marque Helicobacter pylori Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining gastrointestinal stromal tumor (GIST) and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: DOG1 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply DOG1 (discovered on GIST-1) Control Slides are for the positive immunohistochemical staining of DOG1, expressed predominantly on the plasma membrane of gastrointestinal stromal tumors (GIST).
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply DOG1 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| DOG1 positive expression | Brown membrane & cytoplasmic staining |
| Kidney | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque DOG1 (SP31) is the concentrated primary antibody used. Dilute primary antibody to 1/25 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque DOG1 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining leiomyoma uterus and negative staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Desmin quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Desmin Control Slides are for the positive immunohistochemical staining of Desmin, a protein found in normal smooth, skeletal, and cardiac muscle cells and expressed in leiomyoma, leiomyosarcoma, rhabdomyoma, and rhabdomyosarcoma tumors.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Desmin primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Desmin positive expression | Brown cytoplasmic staining |
| Tonsil | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Desmin (D33) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Desmin Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining lung and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Cytomegalovirus quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Cytomegalovirus (CMV) Control Slides are for the positive immunohistochemical staining of cytomegalovirus, a member of the herpes virus group, including herpes simplex virus types 1 and 2, varicella zoster virus, and Epstein Barr virus.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Cytomegalovirus primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CMV positive expression | Brown nuclear staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CMV (8B1.2, 1G5.2, 2D4.2) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CMV Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining skin and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Cytokeratin, HMW quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Cytokeratin, HMW (High Molecular Weight) Control Slides are for the positive immunohistochemical staining of Cytokeratin, HMW, expressed in squamous, ductal and complex epithelia. Useful in differentiation of benign prostate glands from prostatic adenocarcinoma and classification of neoplastic tissue as carcinoma or epithelial origin.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Cytokeratin, High Molecular Weight primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Link. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Label. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Cytokeratin, HMW positive expression | Brown cytoplasmic staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Agilent Cytokeratin, High Molecular Weight (34ßE12) is the pre-dilute primary antibody used.
- Agilent LSAB2 Visualization Kit provides the Link and Label solutions used.
- Cell Marque DAB Substrate Kit is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Agilent Cytokeratin, High Molecular Weight Antibody datasheet.
- Agilent LSAB2 Visualization Kit datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining skin, positive staining colon and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Cytokeratin AE1/AE3 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Cytokeratin AE1/AE3 Control Slides are for the positive immunohistochemical staining of Cytokeratin’s AE1/AE3, present in normal and abnormal tissues, used to distinguish epithelial carcinoma from non-epithelial malignancies and aid in epithelial tumor classification.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Cytokeratin Cocktail (AE1/AE3) primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Cytokeratin AE1/AE3 positive expression | Brown cytoplasmic staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Cytokeratin Cocktail (AE1/AE3) (313M) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Cytokeratin Cocktail (AE1/AE3) Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining colon and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Cytokeratin 20 (CK20) quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Cytokeratin 20 (CK20) Control Slides are for the positive immunohistochemical staining of Cytokeratin 20, expressed in gastric and intestinal epithelium, urothelium and Merkel cells. CK20 has been identified in colon, stomach, pancreas and biliary system adenocarcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Cytokeratin 20 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CK20 positive expression | Brown cytoplasmic staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Cytokeratin 20 (Ks20.8) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Cytokeratin 20 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining pancreas and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Cytokeratin 7 (CK7) quality control stained slide(s) included
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Cytokeratin 7 (CK7) Control Slides are for the positive immunohistochemical staining of CK7, reacting with proteins in ductal, glandular and transitional epithelia of the urinary tract and bile duct. CK7 will also distinguish positive staining lung and breast epithelium from negative staining colon and prostate epithelial cells
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Cytokeratin 7 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CK7 positive expression | Brown cytoplasmic staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Cytokeratin 7 (OV-TL 12/30) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Cytokeratin 7 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Cytokeratin 5/6 (CK5/6) quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Cytokeratin 5/6 (CK5/6) Control Slides are for the positive immunohistochemical staining of CK5/6, a useful marker in identification of mesothelioma and lung squamous cell carcinoma and also expressed in a wide range of normal tissue such as breast, prostate, mesothelium, skin and esophagus.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CK5/6 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CK5/6 positive expression | Brown cytoplasmic staining |
| Liver | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CK5/6 (D5/16B4) is the concentrated primary antibody used. Dilute primary antibody to 1/200 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CK5/6 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining pancreas and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Chromogranin A quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Chromogranin A Control Slides are for the positive immunohistochemical staining of Chromogranin A, expressed in a wide variety of endocrine tissues such as pituitary, pancreas, hypothalamus, and parathyroid and is useful in identification of neuroendocrine tumors.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Chromogranin A primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Chromogranin A positive expression | Brown cytoplasmic staining |
| Kidney | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Chromogranin A (LK2H10) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Chromogranin A Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining colon and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CDX2 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CDX2 Control Slides are for the positive immunohistochemical staining of CDX2, a caudal-related homeobox gene expressed in nuclei of intestinal epithelial cells and in primary and metastatic colorectal carcinomas. CDX2 has also been identified in intestinal metaplasia of the stomach and intestinal-type gastric cancer.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CDX2 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CDX2 positive expression | Brown nuclear staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30% Aqueous Solution (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CDX2 (EPR2764Y) is the concentrated primary antibody used. Dilute primary antibody to 1/500 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CDX2 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD138 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD138 Control Slides are for the positive immunohistochemical staining of CD138, a specific marker for identification of plasma cells and plasma cell differentiation within hematolymphoid tissues in benign and neoplastic conditions.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD138 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP-Polymer solution. Incubate for 20 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD138 positive expression | Brown membranous staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare CD138 (B-A38) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Biocare Van Gogh Yellow Diluent (PD902).
- Biocare Mach 2™ Universal HRP-Polymer Detection (M2U522) is the HRP-Polymer solution used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare CD138 Antibody datasheet.
- Biocare Van Gogh Yellow Diluent datasheet.
- Biocare Mach 2™ Universal HRP-Polymer Detection datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD68 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD68 Control Slides are for the positive immunohistochemical staining of CD68, a useful marker for cells of macrophage lineage, including monocytes and histiocytes. Kupffer cells and osteoclasts are also stained.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD68 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD68 positive expression | Brown cytoplasmic & membrane staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30% Aqueous Solution (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD68 (Kp-1) is the concentrated primary antibody used. Dilute primary antibody to 1/3500 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD68 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining thyroid and negative staining lung
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD56 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD56 Control Slides are for the positive immunohistochemical staining of CD56, expressed in a variety of normal and abnormal tissues. CD56 recognizes two proteins of the neural cell adhesion molecule, the basic molecule expressed on most neuroectodermally derived tissues and neoplasms. It is also expressed on some mesodermally derived tumors.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD56 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD56 positive expression | Brown membrane staining |
| Lung | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD56 (123C3.D5) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD56 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD34 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD34 Control Slides are for the positive immunohistochemical staining of CD34, found in vascular endothelium and expressed by spindle cell tumors, such as solitary fibrous tumors and gastrointestinal stromal tumors (GIST). Useful for differentiation in myeloid and lymphoid neoplasms.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD34 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD34 positive expression | Brown membrane staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD34 (QBEnd/10) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD34 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining Hodgkin’s lymphoma and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD30 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD30 Control Slides are for the positive immunohistochemical staining of CD30, expressed by Reed-Sternberg cells in classic Hodgkin lymphoma, the majority of anaplastic large cell lymphomas, primary cutaneous CD30 positive T-cell lymphoproliferative disorders, and in embryonal carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD30 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD30 positive expression | Brown cellular membrane staining |
| Kidney | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD30 (Ber-H2) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD30 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining GIST (gastrointestinal stromal tumor) and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD117, c-kit quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD117, c-kit Control Slides are for the positive immunohistochemical staining of CD117, found in a wide variety of tumors and useful in classification of tumors of the gastrointestinal tract, mast cell neoplasms and seminomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD117 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD117 positive expression | Brown cytoplasmic & membrane staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD117, c-kit (YR145) is the concentrated primary antibody used. Dilute primary antibody to 1/50 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD117 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD10 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD10 Control Slides are for the positive immunohistochemical staining of CD10, also known as Common Acute Lymphoblastic Leukemia Antigen (CALLA), useful in the classification of B-cell leukemias and lymphomas and identification of hepatocellular and renal cell carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD10 primary antibody. Incubate at room temperature for 30 to 60 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP-Polymer solution. Incubate for 20 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD10 positive expression | Brown cytoplasmic & membrane staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Biocare CD10 (56C6) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Biocare Renoir Red Diluent (PD904).
- Biocare Mach 2™ Universal HRP-Polymer Detection (M2U522) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Biocare CD10 Antibody datasheet.
- Biocare Renoir Red Diluent datasheet.
- Biocare Mach 2™ Universal HRP-Polymer Detection datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD8 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD8 Control Slides are for the positive immunohistochemical staining of CD8, a useful marker for the detection of T-cell involvement in cytotoxic immunoreactions and classification of lymphocytes and malignant lymphomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD8 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CD8 positive expression | Brown membranous staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD8 (C8/144B) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD8 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining colon and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Carcinoembryonic Antigen (CEA) quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Carcinoembryonic Antigen (CEA) Control Slides are for the positive immunohistochemical staining of CEA, useful in distinguishing mesothelioma from adenocarcinoma. Many adenocarcinomas show diffuse strong positive staining for CEA, while mesotheliomas typically fail to stain or show rare weakly positive cells.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CEA primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| CEA positive expression | Brown cytoplasmic staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CEA (polyclonal 236A) is the concentrated primary antibody used. Dilute primary antibody to 1/400 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CEA Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining mesothelioma and negative staining myometrium.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Calretinin quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Calretinin Control Slides are for the positive immunohistochemical staining of Calretinin, useful in differentiating mesothelioma from adenocarcinomas of the lung and other sources.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Calretinin primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Calretinin positive expression | Brown cytoplasmic & nuclear staining |
| Myometrium | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Calretinin (polyclonal 232A) is the concentrated primary antibody used. Dilute primary antibody to 1/600 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Calretinin Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver and negative staining adipose.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alpha-Fetoprotein (AFP) quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply, Alpha-Fetoprotein (AFP) Control Slides are for the positive immunohistochemical staining of Alpha-Fetoprotein, a secretory protein expressed in liver carcinomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #1.
-
- Proceed with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
-
- See Procedure Note #2.
-
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
-
- See Procedure Note #3.
-
- Tap off excess buffer; apply Alpha-Fetoprotein primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain with Hematoxylin Stain, Gill I (Part 1180); 1-10 dips.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| AFP positive expression | Brown cytoplasmic staining |
| Adipose | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
-
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Alpha-Fetoprotein Polyclonal is the primary antibody used along with Cell Marque detection and ancillary reagents.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Cell Marque Alpha-Fetoprotein Antibody datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: BCL6 quality control stained slide(s) included
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply BCL6 (B-cell lymphoma 6) Control Slides are for the positive immunohistochemical staining of BCL6, commonly expressed in diffuse large cell lymphomas, follicular lymphomas and Burkitt’s lymphoma.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply BCL6 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| BCL6 positive expression | Brown nuclear staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque BCL6 (GI191/A8) is the concentrated primary antibody used. Dilute primary antibody to 1/400 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque BCL6 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining tonsil and negative staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: CD20 quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply CD20, B-Cell Control Slides are for the positive immunohistochemical staining of normal and neoplastic B-cells, generally located in follicles of lymph nodes and tonsils. CD20 is considered to be a pan-B-cell marker, occasionally detected in T-cell malignancies and a very strong marker of mature B-cell lymphomas.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply CD20 primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| B-cell positive expression | Brown cytoplasmic staining |
| Kidney | Negative |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque CD20 (L26) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque CD20 Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining uterus
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Actin, Smooth Muscle quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
APPLICATION:
Newcomer Supply Actin, Smooth Muscle, Control Slides are for the positive immunohistochemical staining of smooth muscle actin. Myofibroblasts and myoepithelial cells will also positively react.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #1.
- Proceed, if necessary, with an epitope/antigen retrieval technique approved for use in your laboratory.
- Rinse in distilled water; tap off excess water.
- Circle sections with Pap Pen Liquid Blocker (Part 6505, 6506 or 6507) to reduce reagent usage and ensure tissue coverage.
- Block endogenous peroxidase with freshly made 3% Hydrogen Peroxide. Incubate for 5 minutes.
- See Procedure Note #2.
- Wash slides gently in distilled water. Rinse in two changes of Tris Buffered Saline.
- See Procedure Note #3.
- Tap off excess buffer; apply Actin, Smooth Muscle primary antibody. Incubate at room temperature for 30 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply Amplifier. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Tap off excess buffer; apply HRP Polymer. Incubate for 10 minutes.
- Rinse slides in two changes of buffer.
- Prepare required quantity of DAB substrate/chromogen.
- Tap off excess buffer; apply DAB. Incubate for 5 minutes.
- Rinse slides in four changes of distilled water.
- Counterstain lightly with Hematoxylin Stain, Gill I (Part 1180) for 5 minutes.
- Rinse slides in warm tap water to blue sections.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Actin, Smooth Muscle positive expression | Brown cytoplasmic staining |
| Nuclei | Blue |
PROCEDURE NOTES:
- Do not allow sections to dry out at any point during procedure.
- Dilute sufficient Hydrogen Peroxide 30%, Aqueous (Part 1206) with distilled water to a 3% (1/10) solution prior to use.
- Dilute sufficient Tris Buffered Saline 0.05M, pH 7.6, 10X (Part 140304) with distilled water to a 1/10 solution prior to use for all buffer rinses in this procedure.
- Cell Marque Actin, Smooth Muscle (1A4) is the concentrated primary antibody used. Dilute primary antibody to 1/100 working dilution with Cell Marque Emerald: Antibody Diluent (936B).
- Cell Marque HiDef Detection™ HRP Polymer System (954D) provides the Amplifier and HRP Polymer solutions used.
- Cell Marque DAB Substrate Kit (957D) is the chromogen used.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Cell Marque Actin, Smooth Muscle Antibody datasheet.
- Cell Marque Emerald: Antibody Diluent datasheet.
- Cell Marque HiDef Detection™ Polymer System datasheet.
- Cell Marque DAB Substrate Kit datasheet.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining gouty tophi tissue.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Urate Stain, Gomori Methenamine Silver Method: | Individual Stain Solution |
| Methenamine 3%, Aqueous | Part 12239 |
| Silver Nitrate 5%, Aqueous | Part 13805 |
| Sodium Borate 5%, Aqueous | Part 13826 |
| Gold Chloride 0.25%, Aqueous | Part 11287 |
| Sodium Thiosulfate 2.5%, Aqueous | Part 13889 |
| Light Green SF Yellowish Stain 0.2%, Aqueous | Part 12202 |
APPLICATION:
Newcomer Supply Urates Control Slides are for the positive histochemical staining of urates in tissue sections. With abnormal accumulations found around joints and in soft tissues, this disturbance in uric acid metabolism is known as gout, with collections of urate crystals referred to as gouty tophi.
Calcium pyrophosphate crystals or pseudogout may also be demonstrated in this procedure. When viewed with a polarizing filter and red compensator filter, gout and pseudogout can be distinguished.
Fixation: Urate crystals are soluble in aqueous solutions. Fix in 100% ethyl alcohol; a minimum of two changes, 4 hours each.
Processing: Transfer from 100% ethyl alcohol fixative to xylene for 1 hour; proceed with equal parts xylene/paraffin at 58°C for 2 hours. Infiltrate with paraffin for a minimum of 1 hour; embed.
Technique: Chill paraffin blocks in 100% ethyl alcohol; cut paraffin sections with minimal water bath exposure.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Prepare Methenamine Silver Stock Solution.
- Methenamine 3%, Aqueous 50 ml
- Silver Nitrate 5%, Aqueous 2.5 ml
- Slowly add silver nitrate; mix to clear milky precipitate.
- Store clear solution at 2°C-8°C for up to 2 months.
- Prepare fresh Methenamine Silver Working Solution; combine and mix well.
- Methenamine Silver Stock Solution 25 ml
- Distilled Water 25 ml
- Sodium Borate 5%, Aqueous 3 ml
- Preheat fresh Methenamine Silver Working Solution to 60°C in a water bath.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Rinse in two changes of 100% ethyl alcohol, 10 dips each.
- Do not use 95% ethyl alcohol or distilled water steps.
- See Procedure Notes #3 and #4.
- Incubate slides in preheated Methenamine Silver Working Solution (Step #5) in a 60°C water bath for 30 minutes.
- Remove control slide, rinse in warm distilled water, check microscopically for adequate silver development. Crystals should be dark brown/black.
- If structures are not sufficiently dark, place slides back in warm silver solution.
- Recheck at 2-3 minute intervals until desired intensity is achieved.
- Rinse well in distilled water.
- Tone in Gold Chloride 0.25%, Aqueous until brown colorization disappears; 5 to 30 seconds.
- Rinse well in distilled water.
- Place in Sodium Thiosulfate 2.5%, Aqueous; 2 to 3 minutes.
- Wash well in running tap water for 3 minutes; rinse in distilled water.
- Counterstain in Light Green SF Yellowish Stain 0.2%, Aqueous for 1 to 2 minutes, depending on preference of counterstain intensity.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Light Field Microscopy: | ||
| Gout/urate crystals | Black | |
| Background | Green | |
| Polarized/Red Compensator Filter: (long axes aligned parallel) | ||
| Gout/urate crystals | Yellow, long & needle shaped | |
| Pseudogout crystals | Blue, shorter & rhomboidal | |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.255-256, 267-268.
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 187-188.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 225-226.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining uterus.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori One-Step Aniline Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Trichrome, Gomori One-Step, Aniline Blue Stain Kit: | Part 9176B/A | Individual Stain Solution | |
| Solution A: | Bouin Fluid | 250/500 ml | Part 1020 |
| Solution B: | Ferric Chloride, Acidified | 125/250 ml | Part 1409 |
| Solution C: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution D: | Trichrome Stain, Gomori One-Step, Aniline Blue | 250/500 ml | Part 1403 |
| Solution E: | Acetic Acid 0.5%, Aqueous | 250/500 ml | Part 100121 |
APPLICATION:
Newcomer Supply Trichrome, Uterus Control Slides are for the positive histochemical staining of connective tissue and to differentially demonstrate collagen and muscle fibers.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Preheat Solution A: Bouin Fluid to 56-60°C in oven or water bath. (Skip if using overnight method or microwave procedure.)
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Mordant in preheated Solution A: Bouin Fluid (Step #2) for one hour at 56-60°C or overnight at room temperature. Cool at room temperature for 5-10 minutes.
- Skip Step #4 if tissue was originally Bouin fixed.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing Solution A: Bouin Fluid and microwave for 5 minutes at 60°C.
- Wash well in running tap water; rinse in distilled water.
- Prepare fresh Weigert Iron Hematoxylin; combine and mix well.
- Solution B: Ferric Chloride, Acidified 20 ml
- Solution C: Hematoxylin 1%, Alcoholic 20 ml
- Stain in fresh Weigert Iron Hematoxylin for 10 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #4.
- Stain with Solution D: Trichrome Stain, Gomori One-Step, Aniline Blue for 20 minutes.
- Differentiate in Solution E: Acetic Acid 0.5%, Aqueous; 2 minutes.
- Rinse quickly in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Collagen and mucin | Blue |
| Muscle fibers, cytoplasm and keratin | Red |
| Nuclei | Blue/black |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If Weigert Iron Hematoxylin is not completely washed from tissue sections, nuclear and cytoplasmic staining may be compromised.
- Trichrome, Uterus Control Slides are validated with Trichrome Stain Kit, Gomori One-Step, Aniline Blue but can be used as positive controls with any preferred Trichrome procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Brown, Richard. Histologic Preparations: Common Problems and Their Solutions. Northfield, Ill.: College of American Pathologists, 2009. 95-101.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 165-166.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 191-192.
- Vacca, Linda L. Laboratory Manual of Histochemistry. New York: Raven Press, 1985. 308-310.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver, positive staining kidney and positive staining uterus.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori One-Step Aniline Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Trichrome, Gomori One-Step, Aniline Blue Stain Kit: | Part 9176B/A | Individual Stain Solution | |
| Solution A: | Bouin Fluid | 250/500 ml | Part 1020 |
| Solution B: | Ferric Chloride, Acidified | 125/250 ml | Part 1409 |
| Solution C: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution D: | Trichrome Stain, Gomori One-Step, Aniline Blue | 250/500 ml | Part 1403 |
| Solution E: | Acetic Acid 0.5%, Aqueous | 250/500 ml | Part 100121 |
APPLICATION:
Newcomer Supply Trichrome, Multi-Tissue Control Slides, use a combination of tissue sources for the positive histochemical staining of connective tissue and to differentially demonstrate collagen and muscle fibers.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Preheat Solution A: Bouin Fluid to 56-60°C in oven or water bath. (Skip if using overnight method or microwave procedure.)
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Mordant in preheated Solution A: Bouin Fluid (Step #2) for one hour at 56-60°C or overnight at room temperature. Cool at room temperature for 5-10 minutes.
- Skip Step #4 if tissue was originally Bouin fixed.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing Solution A: Bouin Fluid and microwave for 5 minutes at 60°C.
- Wash well in running tap water; rinse in distilled water.
- Prepare fresh Weigert Iron Hematoxylin; combine and mix well.
- Solution B: Ferric Chloride, Acidified 20 ml
- Solution C: Hematoxylin 1%, Alcoholic 20 ml
- Stain in fresh Weigert Iron Hematoxylin for 10 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #4.
- Stain with Solution D: Trichrome Stain, Gomori One-Step, Aniline Blue for 20 minutes.
- Differentiate in Solution E: Acetic Acid 0.5%, Aqueous; 2 minutes.
- Rinse quickly in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Collagen and mucin | Blue |
| Muscle fibers, cytoplasm and keratin | Red |
| Nuclei | Blue/black |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If Weigert Iron Hematoxylin is not completely washed from tissue sections, nuclear and cytoplasmic staining may be compromised.
- Trichrome, Multi-Tissue Control Slides are validated with Trichrome Stain Kit, Gomori One-Step, Aniline Blue but can be used as positive controls with any preferred Trichrome procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Brown, Richard. Histologic Preparations: Common Problems and Their Solutions. Northfield, Ill.: College of American Pathologists, 2009. 95-101.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 165-166.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 191-192.
- Vacca, Linda L. Laboratory Manual of Histochemistry. New York: Raven Press, 1985. 308-310.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori One-Step Aniline Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Trichrome, Gomori One-Step, Aniline Blue Stain Kit: | Part 9176A/B | Individual Stain Solution | |
| Solution A: | Bouin Fluid | 250/500 ml | Part 1020 |
| Solution B: | Ferric Chloride, Aqueous | 125/250 ml | Part 1409 |
| Solution C: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution D: | Trichrome Stain, Gomori One-Step, Aniline Blue | 250/500 ml | Part 1403 |
| Solution E: | Acetic Acid 0.5%, Aqueous | 250/500 ml | Part 100121 |
APPLICATION:
Newcomer Supply Trichrome, Liver Control Slides are for the positive histochemical staining of connective tissue and to differentially demonstrate collagen and muscle fibers.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Preheat Solution A: Bouin Fluid to 56-60°C in oven or water bath. (Skip if using overnight method or microwave procedure.)
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Mordant in preheated Solution A: Bouin Fluid (Step #2) for one hour at 56-60°C or overnight at room temperature. Cool at room temperature for 5-10 minutes.
- Skip Step #4 if tissue was originally Bouin fixed.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing Solution A: Bouin Fluid and microwave for 5 minutes at 60°C.
- Wash well in running tap water; rinse in distilled water.
- Prepare fresh Weigert Iron Hematoxylin; combine and mix well.
- Solution B: Ferric Chloride, Aqueous 20 ml
- Solution C: Hematoxylin 1%, Alcoholic 20 ml
- Stain in fresh Weigert Iron Hematoxylin for 10 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #4.
- Stain with Solution D: Trichrome Stain, Gomori One-Step, Aniline Blue for 20 minutes.
- Differentiate in Solution E: Acetic Acid 0.5%, Aqueous; 2 minutes.
- Rinse quickly in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Collagen and mucin | Blue |
| Muscle fibers, cytoplasm and keratin | Red |
| Nuclei | Blue/black |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If Weigert Iron Hematoxylin is not completely washed from tissue sections, nuclear and cytoplasmic staining may be compromised.
- Trichrome, Liver Control Slides are validated with Trichrome Stain Kit, Gomori One-Step, Aniline Blue but can be used as positive controls with any preferred Trichrome procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Brown, Richard. Histologic Preparations: Common Problems and Their Solutions. Northfield, Ill.: College of American Pathologists, 2009. 95-101.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 165-166.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 191-192.
- Vacca, Linda L. Laboratory Manual of Histochemistry. New York: Raven Press, 1985. 308-310.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining kidney.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori One-Step Aniline Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Trichrome, Gomori One-Step, Aniline Blue Stain Kit: | Part 9176B/A | Individual Stain Solution | |
| Solution A: | Bouin Fluid | 250/500 ml | Part 1020 |
| Solution B: | Ferric Chloride, Acidified | 125/250 ml | Part 1409 |
| Solution C: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution D: | Trichrome Stain, Gomori One-Step, Aniline Blue | 250/500 ml | Part 1403 |
| Solution E: | Acetic Acid 0.5%, Aqueous | 250/500 ml | Part 100121 |
APPLICATION:
Newcomer Supply Trichrome, Kidney Control Slides are for the positive histochemical staining of connective tissue and to differentially demonstrate collagen and muscle fibers.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Preheat Solution A: Bouin Fluid to 56-60°C in oven or water bath. (Skip if using overnight method or microwave procedure.)
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Mordant in preheated Solution A: Bouin Fluid (Step #2) for one hour at 56-60°C or overnight at room temperature. Cool at room temperature for 5-10 minutes.
- Skip Step #4 if tissue was originally Bouin fixed.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing Solution A: Bouin Fluid and microwave for 5 minutes at 60°C.
- Wash well in running tap water; rinse in distilled water.
- Prepare fresh Weigert Iron Hematoxylin; combine and mix well.
- Solution B: Ferric Chloride, Acidified 20 ml
- Solution C: Hematoxylin 1%, Alcoholic 20 ml
- Stain in fresh Weigert Iron Hematoxylin for 10 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #4.
- Stain with Solution D: Trichrome Stain, Gomori One-Step, Aniline Blue for 20 minutes.
- Differentiate in Solution E: Acetic Acid 0.5%, Aqueous; 2 minutes.
- Rinse quickly in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Collagen and mucin | Blue |
| Muscle fibers, cytoplasm and keratin | Red |
| Nuclei | Blue/black |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If Weigert Iron Hematoxylin is not completely washed from tissue sections, nuclear and cytoplasmic staining may be compromised.
- Trichrome, Kidney Control Slides are validated with Trichrome Stain Kit, Gomori One-Step, Aniline Blue but can be used as positive controls with any preferred Trichrome procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Brown, Richard. Histologic Preparations: Common Problems and Their Solutions. Northfield, Ill.: College of American Pathologists, 2009. 95-101.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 165-166.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 191-192.
- Vacca, Linda L. Laboratory Manual of Histochemistry. New York: Raven Press, 1985. 308-310.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Steiner-Steiner Modified quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Steiner-Steiner Modified Silver Stain Kit: | Part 9171A | Individual Stain Solution | |
| Solution A: | Uranyl Nitrate 1%, Aqueous | 250 ml | Part 14036 |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | Part 13804 |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 350 ml | Part 1145 |
| Ingredient D: | Hydroquinone, Powder | 5 grams | Part 12089 |
APPLICATION:
Newcomer Supply Spirochete, Animal Control Slides are for the positive histochemical staining of spirochetes, the causative agent of a variety of diseases such as; syphilis, bejel, pinta, yaws and lyme.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Preheat Solution A: Uranyl Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #9.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #11.
- Prepare Hydroquinone Solution; combine and mix well.
-
- Ingredient D: Hydroquinone, Powder 5 gm (or one rounded scoop with reusable mini sampling spoon)
- Distilled Water 25 ml
-
-
- Prepare fresh Reducing Solution by combining in order listed.
-
- Hydroquinone Solution (Step #5) 25 ml
- Solution C: Gum Mastic 2.5%, Alcoholic 15 ml
- Solution B: Silver Nitrate 1%, Aqueous 6 ml
- Solution will turn milky white after addition of Gum Mastic.
- Preheat solution in 45°C water bath. Save for Step #15.
-
- Do not preheat solutions if using Microwave Modifications.
- Prepare fresh Reducing Solution by combining in order listed.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #3.
-
- Sensitize in preheated Solution A: Uranyl Nitrate 1%, Aqueous (Step #3) for 10 minutes in a 60°C water bath.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #4.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Uranyl Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
-
- Rinse well in several changes of distilled water.
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in a 60°C water bath for 15 minutes.
-
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Remove from microwave, cover and let sit for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
-
- Excessive rinsing may cause nucleus to pick up silver.
-
- Rinse well in several changes of distilled water.
-
- Dip 5 times in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 3 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45°C water bath for 10-30 minutes with frequent agitation. Examine microscopically after 10 minutes of incubation.
-
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2-5 minutes until desired result is achieved.
-
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 1 minute.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 1 minute until desired results are achieved.
-
-
-
- Directly dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Spirochetes | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Garvey, Winsome. “Some Favorite Silver Stains.” The Journal of Histotechnology 3 (1996): 269-278.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 218-219.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Swisher, Billie. “Modified Steiner Procedure for Microwave Staining of Spirochetes and Nonfilamentous Bacteria.” The Journal of Histotechnology4 (1987): 241-243.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Steiner-Steiner Modified quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Steiner-Steiner Modified Silver Stain Kit: | Part 9171A | Individual Stain Solution | |
| Solution A: | Uranyl Nitrate 1%, Aqueous | 250 ml | Part 14036 |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | Part 13804 |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 350 ml | Part 1145 |
| Ingredient D: | Hydroquinone, Powder | 5 grams | Part 12089 |
APPLICATION:
Newcomer Supply Spirochete, Artificial Control Slides are for the positive histochemical staining of spirochetes, the causative agent of a variety of diseases such as; syphilis, bejel, pinta, yaws and lyme. Brachyspira hyodysenteriae, purchased from American Type Culture Collection, is used to produce the positive control tissue.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Preheat Solution A: Uranyl Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #9.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #11.
- Prepare Hydroquinone Solution; combine and mix well.
- Ingredient D: Hydroquinone, Powder 0.5 gm
(or one rounded scoop with reusable mini sampling spoon)
- Distilled Water 25 ml
- Prepare fresh Reducing Solution by combining in order listed.
- Hydroquinone Solution (Step #5) 25 ml
- Solution C: Gum Mastic 2.5%, Alcoholic 15 ml
- Solution B: Silver Nitrate 1%, Aqueous 0.6 ml
- Solution will turn milky white after addition of Gum Mastic.
- Preheat solution in 45°C water bath. Save for Step #15.
- Do not preheat solutions if using Microwave Modifications.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Note #3
- Sensitize in preheated Solution A: Uranyl Nitrate 1%, Aqueous (Step #3) for 10 minutes in a 60°C water bath.
Microwave Modification: See Procedure Note #4.
- Place slides in a plastic Coplin jar with Solution A: Uranyl Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Rinse well in several changes of distilled water.
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in a 60°C water bath for 15 minutes.
Microwave Modification:
- Place slides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Remove from microwave, cover and let sit for 1 minute.
- Rinse well in several changes of distilled water.
- Excessive rinsing may cause nucleus to pick up silver.
- Dip 5 times in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 5 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45°C water bath for 10-30 minutes with frequent agitation. Examine microscopically after 10 minutes of incubation.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2-5 minutes until desired results are achieved.
Microwave Modification:
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 1 minute.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 1 minute until desired results are achieved.
- Directly dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Spirochetes | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Garvey, Winsome. “Some Favorite Silver Stains.” The Journal of Histotechnology 3 (1996): 269-278.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 218-219.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Swisher, Billie. “Modified Steiner Procedure for Microwave Staining of Spirochetes and Nonfilamentous Bacteria.” The Journal of Histotechnology4 (1987): 241-243.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining striated muscle.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PTAH quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Phosphotungstic Acid Hematoxylin (PTAH) Stain Kit: | Part 9111A | Individual Stain Solution | |
| Solution A: | Zenker Fixative, Modified, Zinc Chloride | 250 ml | Part 1461 |
| Solution B: | Acetic Acid, Glacial, ACS | 25 ml | Part 10010 |
| Solution C: | Potassium Permanganate 0.25%, Aqueous | 250 ml | Part 133931 |
| Solution D: | Oxalic Acid 5%, Aqueous | 250 ml | Part 1293 |
| Solution E: | Phosphotungstic Acid Hematoxylin (PTAH) Stain, Modified Mallory | 250 ml | Part 1334 |
APPLICATION:
The Newcomer Supply Phosphotungstic Acid Hematoxylin (PTAH) Control Slides are for the positive histochemical demonstration of muscle striations and collagen in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Prepare Zenker Fixative Working Solution; combine and mix well.
Solution A: Zenker Fixative, Modified, Zinc Chloride 38 ml
Solution B: Acetic Acid, Glacial, ACS 2 ml
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Fix in Zenker Fixative Working Solution (Step #2) at 56°C; 3 hours.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing prepared Zenker Fixative Working Solution and microwave for 5 minutes at 60°C.
- Wash well in three changes of tap water; rinse in distilled water.
- Place in Solution C: Potassium Permanganate 0.25%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Solution D: Oxalic Acid 5%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Stain in Solution E: PTAH Stain, Modified Mallory for 12-24 hours at room temperature, or 2 hours at 56°C.
- See Procedure Note #4.
Microwave Modification:
- Place slides in a plastic Coplin jar containing Solution E: PTAH Stain, Modified Mallory and microwave for 7 minutes at 70°C.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Dehydrate quickly, alcohol may extract stain from sections.
RESULTS:
| Muscle striations, fibrin, keratin | Dark blue |
| Collagen, cartilage, elastic fibers | Deep reddish brown |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- This PTAH Stain formulation is twice as strong as the original Mallory formulation; adjust staining time according to preference of intensity. Suggested staining time at 37°C is 18 hours.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008.130-131.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 178-180, 201-202.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 193-194.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining colon.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Mayer Mucicarmine quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Mucin, Mayer Mucicarmine Stain Kit: | Part 9151A/B | Individual Stain Solution | |
| Solution A: | Ferric Chloride, Aqueous | 125/250 ml | Part 1409 |
| Solution B: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution C: | Mucicarmine Stock Stain, Mayer | 125/125 ml | Part 1250 |
| Solution D: | Metanil Yellow Stain, Aqueous | 250/500 ml | Part 12235 |
APPLICATION:
Newcomer Supply Mucin Mucicarmine, Control Slides are for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin).
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Prepare fresh Weigert Iron Hematoxylin Working Solution directly before use; combine and mix well:
- Solution A: Ferric Chloride, Aqueous 20 ml
- Solution B: Hematoxylin 1%, Alcoholic 20 ml
- Stain in fresh Weigert Iron Hematoxylin Working Solution for 7 minutes.
- Rinse in running tap water for 10 minutes.
- Prepare fresh Mayer Mucicarmine Working Solution; combine and mix well:
- Solution C: Mucicarmine Stock Stain, Mayer 10 ml
- Tap Water (do not use distilled water) 30 ml
- Stain in fresh Mayer Mucicarmine Working Solution for 60 minutes or longer if a more intense stain is desired.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing fresh Mayer Mucicarmine Working Solution and microwave at 70°C for 10 minutes.
- Rinse in several changes of tap water.
- Counterstain in Solution D: Metanil Yellow Stain, Aqueous for 30 to 60 seconds.
- Dehydrate quickly through 95% and 100% ethyl alcohols. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Deep rose to red |
| Nuclei | Black |
| Other tissue elements | Yellow |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 174-175.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 142-144.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 168-169.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine and positive staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Movat-Russell Pentachrome quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Movat-Russell Modified Pentachrome Stain Kit: | Part 9150A | Individual Stain Solution | |
| Solution A: | Alcian Blue Stain 1%, Aqueous | 250 ml | |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 50 ml | Part 1006 |
| Solution C: | Hematoxylin 10%, Alcoholic | 100 ml | |
| Solution D: | Ferric Chloride 10%, Aqueous | 100 ml | Part 10856 |
| Solution E: | Iodine, Verhoeff, Aqueous | 100 ml | Part 1209 |
| Solution F: | Ferric Chloride 2%, Aqueous | 250 ml | Part 108553 |
| Solution G: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution H: | Crocein Scarlet 7B Stain, Aqueous | 250 ml | |
| Solution I: | Acid Fuchsin Stain, Aqueous | 100 ml | |
| Solution J: | Phosphotungstic Acid 5%, Aqueous | 500 ml | Part 13345 |
| Solution K: | Orange G Stain 1%, Aqueous | 250 ml | |
| Solution L: | Acetic Acid 0.5%, Aqueous | 500 ml | Part 100121 |
APPLICATION:
Newcomer Supply Movat-Russell Pentachrome Control Slides are for the positive histochemical staining of connective tissue elements, mucin, fibrin, elastic fibers, muscle, and collagen.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Stain in Solution A: Alcian Blue Stain 1%, Aqueous for 20 minutes.
- Wash in running tap water for 5 minutes.
- Prepare fresh Alkaline Alcohol Solution; combine and mix well.
- Solution B: Ammonium Hydroxide 28-30% 5 ml
- Alcohol, Ethyl Denatured, 95% (Part 10842) 45 ml
- Place slides in fresh Alkaline Alcohol Solution for 30 minutes.
- Wash in running tap water for 10 minutes; rinse in distilled water.
- See Procedure Note #3.
- Prepare fresh Hematoxylin Working Stain Solution just before use in the order given; combine and mix well.
- Solution C: Hematoxylin 10%, Alcoholic 10 ml
- Alcohol, Ethyl Denatured 100% (Part 10841) 10 ml
- Solution D: Ferric Chloride 10%, Aqueous 10 ml
- Solution E: Iodine, Verhoeff, Aqueous 10 ml
- Stain in fresh Hematoxylin Working Stain Solution for 15 minutes.
- Discard after successful differentiation in Step #11.
- Rinse in several changes of distilled water.
- Differentiate one slide at a time in Solution F: Ferric Chloride 2%, Aqueous until elastic fibers contrast sharply with the background; approximately 5-10 dips.
- See Procedure Note #4.
- Rinse in distilled water.
- Place in Solution G: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Prepare Crocein Scarlet-Acid Fuchsin Solution:
- Solution H: Crocein Scarlet 7B Stain, Aqueous 40 ml
- Solution I: Acid Fuchsin Stain, Aqueous 10 ml
- Stain in Crocein Scarlet-Acid Fuchsin Solution for 1 minute.
- Rinse in several changes of distilled water.
- Rinse in Solution L: Acetic Acid 0.5%, Aqueous for 30 seconds.
- Place in Solution J: Phosphotungstic Acid 5%, Aqueous; two changes of 5 minutes each.
- Rinse in Solution L: Acetic Acid 0.5%, Aqueous.
- Stain in Solution K: Orange G Stain 1%, Aqueous for 15 minutes.
- Dehydrate through three changes of 100% ethyl alcohol, 10 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Do not use 95% ethyl alcohol in the dehydration step.
RESULTS:
| Nuclei and elastic fibers | Black |
| Collagen and reticular fibers | Yellow |
| Ground substance and mucin | Blue |
| Fibrinoid, fibrin | Intense red |
| Muscle | Red |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- It is important to completely remove Alkaline Alcohol Solution with running tap water. Failure to do so will inhibit the subsequent staining steps.
- Do not over-differentiate in Solution F: Ferric Chloride 2%, Aqueous. If the background is completely colorless, the section may be over-differentiated. Over-differentiated sections may be restained in Hematoxylin Working Stain Solution (Step #9) provided sections have not been treated with an alcohol/dehydration step.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 172-174.
- Movat, Henry, “Demonstration of All Connective Tissue Elements in a Single Section”. AMA Archives of Pathology. 1955; 60 (3): 289–295.
- Russell H. K. Jr. “A Modification of Movat’s Pentachrome Stain”. AMA Archives of Pathology.1972; 94 (2): 187–191.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining skin.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Fontana Masson quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fontana Masson Stain Kit: | Part 9105A | Individual Stain Solution | |
| Solution A: | Silver Nitrate 10%, Aqueous | 250 ml | Part 13806 |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 250 ml | Part 1006 |
| Solution C: | Gold Chloride 0.2%, Aqueous | 250 ml | Part 11286 |
| Solution D: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution E: | Nuclear Fast Red Stain, Kernechtrot | 250 ml | Part 1255 |
APPLICATION:
Newcomer Supply Melanin Control Slides are for the positive histochemical staining of melanin in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Prepare Fontana Silver Working Solution (diamine silver) in an acid cleaned Erlenmeyer flask:
- Solution A: Silver Nitrate 10%, Aqueous; 25 ml
- Add Solution B: Ammonium Hydroxide 28-30%, ACS drop by drop, mix with swirling motion until solution clouds, then clears. Use caution to not add too much Solution B: Ammonium Hydroxide 28-30%, ACS.
- Add more Solution A: Silver Nitrate 10%, Aqueous drop by drop until clear solution becomes slightly cloudy.
- Let solution stand for 2-4 hours before use.
- For use; after standing, filter the solution. Combine 20 ml of this filtered diamine silver solution with 40 ml of distilled water; 60 ml total.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Immerse slides in the Fontana Silver Working Solution (Step #3) in a 45°C to 60°C water bath for 1 hour.
- Check slides microscopically; remove control, rinse in warm distilled water. Confirm that reaction is complete when granules are dark brown and background is colorless.
- Return to heated Fontana Silver Working Solution for longer incubation if indicated.
- Rinse well in three changes of distilled water.
- Immerse in Solution C: Gold Chloride 0.2%, Aqueous; 10 minutes.
- Rinse well in distilled water.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous; 5 minutes.
- Rinse well in distilled water.
- Counterstain in Solution E: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #5.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Melanin | Black |
| Nuclei | Pink-red |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 286-287.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 276-277.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal tumor.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Toluidine Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Toluidine Blue Stain for Mast Cells: | Individual Stain Solution |
| Toluidine Blue Stain 0.1%, Aqueous | Part 14027 |
APPLICATION:
Newcomer Supply Mast Cell, Animal Control Slides are for the positive histochemical staining of mast cells, cells filled with basophilic granules associated with inflammation and allergic reactions, which stain metachromatically with toluidine blue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Toluidine Blue Stain 0.1%, Aqueous for 10 minutes.
- Rinse well in distilled water.
- Dehydrate quickly through two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- See Procedure Note #3.
RESULTS:
| Mast cells | Deep rose-violet |
| Background | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Metachromasia of mast cell granules is stable and will maintain staining during dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.188.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining bladder.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Toluidine Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Toluidine Blue Stain for Mast Cells: | Individual Stain Solution |
| Toluidine Blue Stain 0.1%, Aqueous | Part 14027 |
APPLICATION:
Newcomer Supply Mast Cell Control Slides are for the positive histochemical staining of mast cells, cells filled with basophilic granules associated with inflammation and allergic reactions, which will stain metachromatically with toluidine blue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Toluidine Blue Stain 0.1%, Aqueous for 10 minutes.
- Rinse well in distilled water.
- Dehydrate quickly through two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- See Procedure Note #3.
RESULTS:
| Mast cells | Deep rose-violet |
| Background | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Metachromasia of mast cell granules is stable and will maintain staining during dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.188.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining spinal cord.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 8 microns on Superfrost™ Plus slides.
Quality Control Stain: Luxol Fast Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Luxol Fast Blue (LFB) Stain Set: | Part 12218A/B | Individual Stain Solution | |
| Solution A: | Luxol Fast Blue Stain 0.1%, Alcoholic | 500/1000 ml | |
| Solution B: | Lithium Carbonate, Saturated Aqueous | 500/1000 ml | Part 12215 |
| Hematoxylin Stain, Harris Modified | Part 1201 | ||
| Acid Alcohol 1% | Part 10011 | ||
APPLICATION:
Newcomer Supply Luxol Fast Blue (LFB) Control Slides are for the positive histochemical staining of myelin sheath in central nervous system tissue and in peripheral nerve. PAS, cresyl violet, hematoxylin and eosin stains can be added to LFB procedure for enhanced staining.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Prepare Working Lithium Carbonate 0.05%; combine and mix well;
-
- Solution B: Lithium Carbonate, Saturated Aqueous 5 ml
- Distilled Water 95 ml
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
-
- Stop at 95% ethyl alcohol; no distilled water rinse.
- See Procedure Notes #1 and #2.
-
- Incubate slides in Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic for 2 hours at 60°C or overnight at 37°C; seal lids tightly.
-
- To enhance stain, add 0.4 ml of Acetic Acid, Glacial, ACS (Part 10010) to 40 ml of Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic before use.
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each.
Microwave Modification: See Procedure Note #3.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Luxol Fast Blue Stain 0.1%, Alcoholic. Microwave at 70°C for 10 minutes.
-
-
-
- Rinse slides quickly in 95% ethyl alcohol; 2-3 dips.
- Rinse slides in distilled water.
- Differentiate slides individually in Working Lithium Carbonate 0.05% (Step #2) for 10-15 seconds with agitation until gray and white matter are colorless and contrast with stained tissue.
- Further differentiate in 70% ethyl alcohol (Part 10844), until gray and white matter can be distinguished. Do not over differentiate.
- Rinse slides in distilled water.
- Check slides microscopically. Continue if additional differentiation is needed. Otherwise proceed directly to Step #12.
-
-
-
- One dip in Lithium Carbonate 0.05%, Aqueous (Step #2).
- Dip in two changes of 70% ethyl alcohol until greenish/blue white matter sharply contrasts with colorless gray matter.
-
-
-
- Rinse thoroughly in distilled water.
- Stain with Hematoxylin Stain, Harris Modified (Part 1201) for 1-5 minutes, depending on preference of intensity.
- Wash in running tap water for 3 minutes.
- Differentiate quickly in Acid Alcohol 1% (Part 10011); 3 dips.
- Wash well in running tap water.
- Blue in Solution B: Lithium Carbonate, Saturated Aqueous.
- Wash well in running tap water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Myelin | Blue to blue/green |
| Nissl substance and nuclei | Blue |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 378.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 206-211.
- Klüver, Heinrich, and Elizabeth Barrera. “A Method for the Combined Staining of Cells and Fibers in the Nervous System.” Journal of Neuropathology and Experimental Neurology4 (1953): 400-403.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining hamster liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Steiner-Steiner Modified quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Steiner-Steiner Modified Silver Stain Kit: | Part 9171A | Individual Stain Solution | |
| Solution A: | Uranyl Nitrate 1%, Aqueous | 250 ml | Part 14036 |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | Part 13804 |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 350 ml | Part 1145 |
| Ingredient D: | Hydroquinone, Powder | 5 grams | Part 12089 |
APPLICATION:
Newcomer Supply Leptospira, Animal Control Slides are for the positive histochemical staining of Leptospira sp, a corkscrew shaped bacterium readily visualized with silver staining techniques.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Preheat Solution A: Uranyl Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #9.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #11.
- Prepare Hydroquinone Solution; combine and mix well.
-
- Ingredient D: Hydroquinone, Powder 5 gm (or one rounded scoop with reusable mini sampling spoon)
- Distilled Water 25 ml
-
-
- Prepare fresh Reducing Solution by combining in order listed.
-
- Hydroquinone Solution (Step #5) 25 ml
- Solution C: Gum Mastic 2.5%, Alcoholic 15 ml
- Solution B: Silver Nitrate 1%, Aqueous 6 ml
- Solution will turn milky white after addition of Gum Mastic.
- Preheat solution in 45°C water bath. Save for Step #15.
-
- Do not preheat solutions if using Microwave Modifications.
- Prepare fresh Reducing Solution by combining in order listed.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #3
-
- Sensitize in preheated Solution A: Uranyl Nitrate 1%, Aqueous (Step #3) for 10 minutes in a 60°C water bath.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #4.
-
-
-
- Place slides in a plastic Coplin jar (Part 5184) with Solution A: Uranyl Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in a 60°C water bath for 15 minutes.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Remove from microwave, cover and let sit for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
-
-
-
- Excessive rinsing may cause nucleus to pick up silver.
-
-
-
- Dip 5 times in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 3 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45°C water bath for 10-30 minutes with frequent agitation. Examine microscopically after 10 minutes of incubation.
-
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2-5 minutes until desired results are achieved.
-
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 1 minute.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 1 minute until desired results are achieved.
-
-
-
- Directly dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Leptospira | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Garvey, Winsome. “Some Favorite Silver Stains.” The Journal of Histotechnology 3 (1996): 269-278.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 218-219.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Swisher, Billie. “Modified Steiner Procedure for Microwave Staining of Spirochetes and Nonfilamentous Bacteria.” The Journal of Histotechnology4 (1987): 241-243.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining liver or spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Gomori Prussian Blue quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Iron, Gomori Prussian Blue Stain Kit: | Part 9136A/B | Individual Stain Solution | |
| Solution A: | Hydrochloric Acid 20%, Aqueous | 125/250 ml | Part 12087 |
| Solution B: | Potassium Ferrocyanide 10%, Aqueous | 125/250 ml | Part 13392 |
| Solution C: | Nuclear Fast Red Stain, Kernechtrot | 250/500 ml | Part 1255 |
APPLICATION:
Newcomer Supply Iron Control Slides are for the positive histochemical staining of ferric iron deposits in tissue sections.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Acid clean glassware prior to use to avoid residual iron staining.
-
- See Procedure Note #1.
-
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #2 and #3.
-
- Prepare fresh Ferrocyanide Working Solution directly before use; combine and mix well.
-
- Solution A: Hydrochloric Acid 20%, Aqueous 20 ml
- Solution B: Potassium Ferrocyanide 10%, Aqueous 20 ml
-
- Place slides in fresh Ferrocyanide Working Solution for 20 minutes.
- Rinse in three changes of tap water; rinse in distilled water.
- Place in Solution C: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
-
- Shake solution well before use; do not filter.
-
- Rinse well in distilled water.
-
- See Procedure Note #4.
-
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Ferric iron deposits | Bright blue |
| Nuclei | Red |
| Cytoplasm | Pink |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps
REFERENCES:
-
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 179-184.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 217-218.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Grocott Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, Grocott Methenamine Silver (GMS) Stain Kit: | Part 9121A/B | Individual Stain Solution | |
| Solution A: | Chromic Acid 5%, Aqueous | 250/500 ml | Part 10341 |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250/500 ml | Part 13821 |
| Solution C: | Silver Nitrate | 125/250 ml | Part 1142 |
| Solution D: | Methenamine Borate | 125/250 ml | Part 1142 |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250/500 ml | Part 11285 |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250/500 ml | Part 13888 |
| Solution G: | Light Green SF Yellowish Stain 0.2%, Aqueous | 250/500 ml | Part 12202 |
APPLICATION:
Newcomer Supply Histoplasma, Animal Control Slides are for the positive histochemical staining of fungal organisms in tissue sections. The morphology of the organisms is consistent with Histoplasma sp.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well.
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath approximately 20 to 30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #6
-
-
-
- Microwave Solution A: Chromic Acid 5%, Aqueous without slides in a plastic Coplin jar (Part 5184) for 1 minute at 60°C. Add slides to heated Solution A and oxidize for 10 minutes.
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash in running tap water for 5 minutes; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation.
Microwave Modification:
-
- Incubate slides in a plastic Coplin jar containing Silver-Methenamine Working Solution and microwave for 5 minutes at 45°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 3-5 minute intervals.
-
- Rinse in three to four changes of distilled water.
-
-
-
-
- Do not use tap water at this step.
-
-
-
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 10-30 seconds.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.2%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Histoplasma | Sharply outlined in black |
| Background | Green |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Maintain solution between 45°C-60°C to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th ed. Chicago: ASCP Press, 2020. 221-226.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: H&E quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Hematoxylin and Eosin (H&E) Stain: | Individual Stain Solution |
| Hematoxylin Stain, Harris Modified OR Hematoxylin Stain, Harris |
Part 1201 OR Part 12013 |
| Acid Alcohol 1% | Part 10011 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Eosin Y Working Solution | Part 1072 |
APPLICATION:
Newcomer Supply Hematoxylin & Eosin (H&E) Control Slides are for the clear demonstration of nuclei, epithelium, connective tissue and muscle, important tissue elements for documentation of high quality nuclear and cytoplasmic staining.
For quality control measures, use H&E Control Slides each day that H&E staining is performed and additionally after any H&E solution change. Run H&E Control Slides prior to staining any diagnostic cases to ensure acceptable H&E staining.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
- Stain with Hematoxylin Stain, Harris Modified or Hematoxylin Stain, Harris for 1 to 5 minutes, depending on preference of nuclear stain intensity.
- Wash well in three changes of tap water.
- Differentiate quickly in Acid Alcohol 1%.
-
- See Procedure Note #3.
- Rinse immediately in three changes of tap water.
- Blue slides in Lithium Carbonate, Saturated Aqueous or Scott Tap Water Substitute for 10 dips.
- Wash in three changes of tap water; rinse in distilled water.
- Drain excess water from slides; proceed to 70% alcohol for 10 dips.
- Counterstain in Eosin Y Working Solution for 30 seconds to 3 minutes, depending on preference of intensity.
- Dehydrate in two changes of 95% ethyl alcohol for 1 minute each and two changes of 100% ethyl alcohol, 10 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Nuclei | Blue |
| Cytoplasm of epithelium, connective tissue, muscle | Shades of pink |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Differentiate to suit preference of nuclear stain intensity.
- Check microscopically to assure hematoxylin intensity is satisfactory.
- Nuclei should be distinct and background light to colorless.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 118-120.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 143-144, 153-154.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining gastrointestinal tract.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Steiner-Steiner Modified quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Steiner-Steiner Modified Silver Stain Kit: | Part 9171A | Individual Stain Solution | |
| Solution A: | Uranyl Nitrate 1%, Aqueous | 250 ml | Part 14036 |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | Part 13804 |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 350 ml | Part 1145 |
| Ingredient D: | Hydroquinone, Powder | 5 grams | Part 12089 |
APPLICATION:
Newcomer Supply Helicobacter Control Slides are for the positive histochemical staining of spiral shaped Helicobacter bacterium, associated with stomach inflammation and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Preheat Solution A: Uranyl Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #9.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #11.
- Prepare Hydroquinone Solution; combine and mix well.
-
- Ingredient D: Hydroquinone, Powder 5 gm (or one rounded scoop with reusable mini sampling spoon)
- Distilled Water 25 ml
-
-
- Prepare fresh Reducing Solution by combining in order listed.
-
- Hydroquinone Solution (Step #5) 25 ml
- Solution C: Gum Mastic 2.5%, Alcoholic 15 ml
- Solution B: Silver Nitrate 1%, Aqueous 6 ml
- Solution will turn milky white after addition of Gum Mastic.
- Preheat solution in 45°C water bath. Save for Step #15.
-
- Do not preheat solutions if using Microwave Modifications.
- Prepare fresh Reducing Solution by combining in order listed.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #3
-
- Sensitize in preheated Solution A: Uranyl Nitrate 1%, Aqueous (Step #3) for 10 minutes in a 60°C water bath.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #4.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Uranyl Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in a 60°C water bath for 15 minutes.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Remove from microwave, cover and let sit for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
-
- Excessive rinsing may cause nucleus to pick up silver.
-
- Rinse well in several changes of distilled water.
-
- Dip 5 times in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 3 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45°C water bath for 10-30 minutes with frequent agitation. Examine microscopically after 10 minutes of incubation.
-
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2-5 minutes until desired results are achieved.
-
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 1 minute.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 1 minute until desired results are achieved.
-
-
-
- Directly dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Helicobacter | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Garvey, Winsome. “Some Favorite Silver Stains.” The Journal of Histotechnology 3 (1996): 269-278.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 218-219.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Swisher, Billie. “Modified Steiner Procedure for Microwave Staining of Spirochetes and Nonfilamentous Bacteria.” The Journal of Histotechnology4 (1987): 241-243.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Thiazine quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Differential Stain, Monochromatic Method: | Individual Stain Solution |
| Thiazine Stain | Part 10522 |
APPLICATION:
Newcomer Supply Helicobacter, Artificial Control Slides are for the positive histochemical staining of spiral shaped Helicobacter bacterium, associated with stomach inflammation and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
Helicobacter pylori purchased from American Type Culture Collection is used to produce the positive control tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- Place slides in Thiazine Stain for 1-4 minutes depending upon staining intensity preference.
- Rinse slides quickly in distilled water; long enough to remove excess stain.
- Allow slides to air-dry in a vertical position.
- Dip dried slides in xylene and coverslip with compatible mounting medium.
-
- See Procedure Note #1.
-
RESULTS:
| Helicobacter | Dark blue |
| Collagen and muscle | Blue |
| Nuclei | Blue |
| Cytoplasm | Varying shades of light blue |
PROCEDURE NOTES:
-
- The elimination of dehydration steps is necessary to retain the dark stain of the organism.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and coverslipping steps.
REFERENCES:
-
- Potvin, Carol. “A Modified Diff-Quik Stain for Helicobacter pylori in Gastrointestinal Biopsies.” Laboratory Medicine6 (1994): 389-391.
- Skipper, Ray, and Don DeStephano. “A Rapid Stain for Campylobacter pylori in Gastrointestinal Tissue Sections Using Diff-Quik.” The Journal of Histotechnology4 (1989): 303-304.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal gastrointestinal tract.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Steiner-Steiner Modified quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Steiner-Steiner Modified Silver Stain Kit: | Part 9171A | Individual Stain Solution | |
| Solution A: | Uranyl Nitrate 1%, Aqueous | 250 ml | Part 14036 |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | Part 13804 |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 350 ml | Part 1145 |
| Ingredient D: | Hydroquinone, Powder | 5 grams | Part 12089 |
APPLICATION:
Newcomer Supply Helicobacter, Animal Control Slides are for the positive histochemical staining of spiral shaped Helicobacter bacterium, associated with stomach inflammation and implicated in the development of gastric malignancy, peptic ulcers, and chronic gastritis.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Preheat Solution A: Uranyl Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #9.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #11.
- Prepare Hydroquinone Solution; combine and mix well.
-
- Ingredient D: Hydroquinone, Powder 5 gm (or one rounded scoop with reusable mini sampling spoon)
- Distilled Water 25 ml
-
-
- Prepare fresh Reducing Solution by combining in order listed.
-
- Hydroquinone Solution (Step #5) 25 ml
- Solution C: Gum Mastic 2.5%, Alcoholic 15 ml
- Solution B: Silver Nitrate 1%, Aqueous 6 ml
- Solution will turn milky white after addition of Gum Mastic.
- Preheat solution in 45°C water bath. Save for Step #15.
-
- Do not preheat solutions if using Microwave Modifications.
- Prepare fresh Reducing Solution by combining in order listed.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #3
-
- Sensitize in preheated Solution A: Uranyl Nitrate 1%, Aqueous (Step #3) for 10 minutes in a 60°C water bath.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #4.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Uranyl Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in a 60°C water bath for 15 minutes.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Remove from microwave, cover and let sit for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
-
- Excessive rinsing may cause nucleus to pick up silver.
-
- Dip 5 times in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 3 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45°C water bath for 10-30 minutes with frequent agitation. Examine microscopically after 10 minutes of incubation.
-
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2-5 minutes until desired results are achieved.
-
- Rinse well in several changes of distilled water.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 1 minute.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 1 minute until desired results are achieved.
-
-
-
- Directly dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Helicobacter | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Garvey, Winsome. “Some Favorite Silver Stains.” The Journal of Histotechnology 3 (1996): 269-278.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 218-219.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Swisher, Billie. “Modified Steiner Procedure for Microwave Staining of Spirochetes and Nonfilamentous Bacteria.” The Journal of Histotechnology4 (1987): 241-243.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: May-Grunwald Giemsa quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With May-Grunwald Giemsa Stain: | Individual Stain Solution |
| Jenner Stock Stain | Part 1210 |
| Giemsa Stock Stain, Wolbach | Part 1121 |
| Alcohol, Methanol Anhydrous, ACS | Part 12236 |
| Acetic Acid 1%, Aqueous | Part 10012 |
APPLICATION:
Newcomer Supply Giemsa Control Slides are for the positive histochemical and differential staining of hematopoietic tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Rinse in two changes of Alcohol, Methanol Anhydrous, ACS (Part 12236); 3 minutes each.
- Prepare fresh Working Jenner Stain Solution just prior to use; combine and mix well.
- Distilled Water 20 ml
- Jenner Stock Stain 20 ml
- Place slides in fresh Working Jenner Stain Solution for 6 minutes.
- Prepare fresh Working Giemsa Stain Solution just prior to use; combine and mix well.
- Distilled Water 47 ml
- Giemsa Stock Stain, Wolbach 3 ml
- Place slides directly into fresh Working Giemsa Stain Solution for 45 minutes.
- Rinse quickly in distilled water.
- Differentiate each slide individually in Acetic Acid 1%, Aqueous (Part 10012); 6-10 dips.
- Check microscopically for well differentiated nuclei.
- Rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Nuclei | Blue/violet |
| Cytoplasm | Pink/rose to lighter blue shades |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The color range of the stained cells may vary depending upon fixation and degree of differentiation.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 121-122.
- Shapiro, Stanley H., and Hilda Laufer. “Observations on Fixation and Staining of Bone Marrow Biopsies.” The Journal of Histotechnology 11.3 (1988): 145-47.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 157.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining Aspergillus rat lung, positive staining Candida rat lung, and positive staining Histoplasma animal tissue.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Grocott Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, Grocott Methenamine Silver (GMS) Stain Kit: | Part 9121A/B | Individual Stain Solution | |
| Solution A: | Chromic Acid 5%, Aqueous | 250/500 ml | Part 10341 |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250/500 ml | Part 13821 |
| Solution C: | Silver Nitrate | 125/250 ml | Part 1142 |
| Solution D: | Methenamine Borate | 125/250 ml | Part 1142 |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250/500 ml | Part 11285 |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250/500 ml | Part 13888 |
| Solution G: | Light Green SF Yellowish Stain 0.2%, Aqueous | 250/500 ml | Part 12202 |
APPLICATION:
Newcomer Supply Fungus, GMS, Multi-Tissue, Artificial Control Slides, use a variety of lung tissue sources for the positive histochemical staining of Aspergillus sp., Candida sp., and organisms exhibiting morphology consistent with Histoplasma sp. in separate tissue sections.
Aspergillus fumigatus and Candida albicans purchased from Remel Microbiology Products are used to produce the positive control tissue.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well:
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath approximately 20 to 30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #6.
-
-
-
- Microwave Solution A: Chromic Acid 5%, Aqueous without slides in a plastic Coplin jar (Part 5184) for 1 minute at 60°C. Add slides to heated Solution A and oxidize for 10 minutes.
-
-
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash for 5 minutes in running tap water; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation.
-
Microwave Modification:
-
-
-
- Incubate slides in a plastic Coplin jar containing Silver Methenamine Working Solution and microwave for 5 minutes at 45°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 3-5 minute intervals.
-
-
-
- Rinse in three to four changes of distilled water.
-
-
-
- Never use tap water at this step.
-
-
-
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 10-30 seconds.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.2%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Aspergillus | Sharply outlined in black |
| Candida | Sharply outlined in black |
| Histoplasma | Sharply outlined in black |
| Background | Green |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Maintain solution between 45°C-60°C to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th Chicago: ASCP Press, 2020. 221-226.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PAS/Light Green quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, PAS/Light Green Stain Kit: | Part 9122A | Individual Stain Solution | |
| Solution A: | Periodic Acid 0.5%, Aqueous | 250 ml | Part 13308 |
| Solution B: | Schiff Reagent, McManus | 250 ml | Part 1371 |
| Solution C: | Light Green SF Yellowish Stain 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
Newcomer Supply Fungus, PAS, Candida, Artificial Control Slides are for the positive histochemical staining of Candida in tissue sections. Candida albicans purchased from Remel Microbiology Products is used to produce the positive control tissue
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Place in Solution A: Periodic Acid 0.5%, Aqueous for 5 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Drain slides of excess water and stain in Solution B: Schiff Reagent, McManus for 20 minutes.
- Wash gently in lukewarm tap water for 10 minutes to allow pink color to develop.
- Counterstain in Solution C: Light Green SF Yellowish Stain 0.1%, Aqueous for 5 seconds.
-
- See Procedure Note #3.
-
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Candida | Red to magenta |
| Background | Pale green |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Increase or decrease staining time in Light Green SF Yellowish Stain 0.1%, Aqueous for preference of counterstain intensity.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 321-323.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PAS/Light Green quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, PAS/Light Green Stain Kit: | Part 9122A | Individual Stain Solution | |
| Solution A: | Periodic Acid 0.5%, Aqueous | 250 ml | Part 13308 |
| Solution B: | Schiff Reagent, McManus | 250 ml | Part 1371 |
| Solution C: | Light Green SF Yellowish Stain 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
Newcomer Supply Fungus, PAS, Aspergillus, Artificial Control Slides are for the positive histochemical staining of Aspergillus in tissue sections. Aspergillus fumigatus purchased from Remel Microbiology Products is used to produce the positive control tissue.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Place in Solution A: Periodic Acid 0.5%, Aqueous for 5 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Drain slides of excess water and stain in Solution B: Schiff Reagent, McManus for 20 minutes.
- Wash gently in lukewarm tap water for 10 minutes to allow pink color to develop.
- Counterstain in Solution C: Light Green SF Yellowish Stain 0.1%, Aqueous for 5 seconds.
-
- See Procedure Note #3.
-
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Aspergillus | Red to magenta |
| Background | Pale green |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Increase or decrease staining time in Light Green SF Yellowish Stain 0.1%, Aqueous for preference of counterstain intensity.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 321-323.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245.
- Modifications developed by Newcomer Supply Laboratory.
Be ready to handle potentially infectious specimens. Dispose of forceps after use to lessen the risk of exposure to disease. Sterile 4 1/2″ forceps are exceptionally economical and are available in individually wrapped sterile packets.
25 Forceps/Pack
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Grocott Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, Grocott Methenamine Silver (GMS) Stain Kit: | Part 9121A/B | Individual Stain Solution | |
| Solution A: | Chromic Acid 5%, Aqueous | 250/500 ml | Part 10341 |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250/500 ml | Part 13821 |
| Solution C: | Silver Nitrate | 125/250 ml | Part 1142 |
| Solution D: | Methenamine Borate | 125/250 ml | Part 1142 |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250/500 ml | Part 11285 |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250/500 ml | Part 13888 |
| Solution G: | Light Green SF Yellowish Stain 0.2%, Aqueous | 250/500 ml | Part 12202 |
APPLICATION:
Newcomer Supply Fungus, GMS, Candida, Artificial Control Slides are for the positive histochemical staining of Candida in tissue sections. Candida albicans purchased from Remel Microbiology Products is used to produce the positive control tissue.
PRESTAINING PREPARATION
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well:
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath approximately 20 to 30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #6.
-
-
-
- Microwave Solution A: Chromic Acid 5%, Aqueous without slides in a plastic Coplin jar (Part 5184) for 1 minute at 60°C. Add slides to heated Solution A and oxidize for 10 minutes.
-
-
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash for 5 minutes in running tap water; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation.
-
Microwave Modification:
-
-
-
- Incubate slides in a plastic Coplin jar containing Silver-Methenamine Working Solution and microwave for 5 minutes at 45°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 3-5 minute intervals.
-
-
-
- Rinse in three to four changes of distilled water.
-
- Never use tap water at this step.
-
- Rinse in three to four changes of distilled water.
-
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 10-30 seconds.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.2%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Candida | Sharply outlined in black |
| Background | Green |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Maintain solution between 45°C-60°C to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th Chicago: ASCP Press, 2020. 221-226.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Grocott Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, Grocott Methenamine Silver (GMS) Stain Kit: | Part 9121A/B | Individual Stain Solution | |
| Solution A: | Chromic Acid 5%, Aqueous | 250/500 ml | Part 10341 |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250/500 ml | Part 13821 |
| Solution C: | Silver Nitrate | 125/250 ml | Part 1142 |
| Solution D: | Methenamine Borate | 125/250 ml | Part 1142 |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250/500 ml | Part 11285 |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250/500 ml | Part 13888 |
| Solution G: | Light Green SF Yellowish Stain 0.2%, Aqueous | 250/500 ml | Part 12202 |
APPLICATION:
Newcomer Supply Fungus, GMS, Aspergillus, Artificial Control Slides are for the positive histochemical staining of Aspergillus in tissue sections. Aspergillus fumigatus purchased from Remel Microbiology Products is used to produce the positive control tissue.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well:
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath approximately 20 to 30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
Microwave Modification: See Procedure Note #6.
-
-
-
- Microwave Solution A: Chromic Acid 5%, Aqueous without slides in a plastic Coplin jar (Part 5184) for 1 minute at 60°C. Add slides to heated Solution A and oxidize for 10 minutes.
-
-
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash for 5 minutes in running tap water; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation.
-
Microwave Modification:
-
-
-
- Incubate slides in a plastic Coplin jar containing Silver Methenamine Working Solution and microwave for 5 minutes at 45°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 3-5 minute intervals.
-
-
-
- Rinse in three to four changes of distilled water.
-
- Never use tap water at this step.
-
- Rinse in three to four changes of distilled water.
-
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 10-30 seconds.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.2%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Aspergillus | Sharply outlined in black |
| Background | Green |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Maintain solution between 45°C-60°C to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th ed. Chicago: ASCP Press, 2020. 221-226.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: AFB, Fite quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With AFB, Fite Stain Kit: | Part 91013A | Individual Stain Solution | |
| Solution A: | Xylene/Peanut Oil, 2:1 | 500 ml | Part 1449 |
| Solution B: | Carbol Fuchsin Stain, Ziehl-Neelsen | 250 ml | Part 1030 |
| Solution C: | Acid Alcohol 1% | 250 ml | Part 10011 |
| Solution D: | Light Green SF Yellowish 0.1%, Aqueous | 250 ml | Part 12203 |
APPLICATION:
Newcomer Supply Fite, Nocardia, Artificial Control Slides are for the positive histochemical staining of Nocardia sp. and acid fast bacilli in tissue sections. Nocardia asteroides, purchased from Remel Microbiology Products, is used to produce the positive control tissue.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Filter Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen with high quality filter paper.
- Prepare Diluted Acid Alcohol Solution:
-
- Solution C: Acid Alcohol 1% 20 ml
- Distilled Water 20 ml
-
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 12 minutes each.
-
- See Procedure Note #1.
-
- Drain slides, wipe off excess oil, and blot to opacity taking care to remove residual oil.
-
- See Procedure Note #2.
-
- Stain in freshly filtered Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature.
- Rinse well in distilled water.
- Differentiate slides individually in Diluted Acid Alcohol Solution (Step #3) until background is pale pink; 10-20 dips.
- Quickly rinse in distilled water and check microscopically for correct differentiation.
- Rinse well in distilled water.
- Counterstain in Solution D: Light Green SF Yellowish 0.1%, Aqueous; 5-10 dips.
- Rinse in distilled water.
- Blot excess water from slide and air-dry or oven-dry completely.
- Dip dried slides in xylene and coverslip with a compatible mounting medium.
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 12 minutes each.
RESULTS:
| Nocardia | Red |
| Acid-fast bacilli | Red |
| Other tissue elements | Green |
PROCEDURE NOTES:
-
- Acid-fastness of the organisms is enhanced when the waxy capsule is protected by the mixture of xylene/peanut oil and the avoidance of dehydrating solutions.
- It is important to blot well, residual oil may produce staining artifact.
- A small percentage of Nocardia sp. organisms may resist taking the red stain and remain green due to the growth phase of the individual organism.
- If using a xylene substitute, follow manufacturer’s recommendation for coverslipping step.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-instructional Text. 5th ed. Chicago: ASCP Press, 2020. 215-216.
- Fite, George, P.J. Cambre and M.H. Turner. “Procedure for Demonstrating Lepra Bacilli in Paraffin Sections”. Archives of Pathology 43 (1947). 624-625.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining skin.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Verhoeff-Van Gieson Elastic quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Elastic, Verhoeff Stain Kit: |
Part 9116A/B | Individual Stain Solution | |
| Solution A: |
Hematoxylin 5%, Alcoholic | 125/250 ml | Part 11623 |
| Solution B: |
Ferric Chloride 10%, Aqueous | 125/250 ml | Part 10856 |
| Solution C: |
Iodine, Lugol’s, Aqueous | 75/150 ml | Part 12092 |
| Solution D: |
Sodium Thiosulfate 5%, Aqueous | 250/500 ml | Part 1389 |
| Solution E: |
Van Gieson Stain | 250/500 ml | Part 1404 |
APPLICATION:
Newcomer Supply Elastic, Skin Control Slides are for the positive histochemical staining of elastic fibers in skin.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Prepare fresh Verhoeff Working Solution by combining in the exact order listed, mixing well after each addition. Save for Step #5.
-
- Solution A: Hematoxylin 5%, Alcoholic 20 ml
- Solution B: Ferric Chloride 10%, Aqueous 8 ml
- Solution C: Iodine, Lugol’s, Aqueous 8 ml
-
- Prepare fresh Ferric Chloride 2%, Aqueous Solution for Step #7.
-
- Solution B: Ferric Chloride 10%, Aqueous 10 ml
- Distilled water 40 ml
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in fresh Verhoeff Working Solution (Step #2) for 20 minutes.
-
- Discard solution after successful differentiation in Step #7.
-
- Rinse in several changes of tap water.
- Differentiate each slide individually in fresh Ferric Chloride 2%, Aqueous Solution (Step #3) with agitation; approximately 20 dips.
- Check differentiation: rinse well in tap water and check microscopically for black elastic staining with gray background.
-
- If necessary, return to Ferric Chloride 2%, Aqueous Solution until desired elastic differentiation is achieved.
- See Procedure Notes #3 and #4.
-
- Wash well in tap water.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash well in running tap water for 5 minutes.
- Counterstain in Solution E: Van Gieson Stain for 3 to 5 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Elastic fibers/tissue/nuclei | Blue-black to black |
| Collagen | Red |
| Other tissue elements | Yellow |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If background is colorless, the section has been over-differentiated.
-
- Over-differentiated sections may be re-stained in Step #5 provided sections have not been treated with alcohol.
-
- Differentiation can vary between slides dependent upon the amount of elastic tissue present in sections.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 167-169.
- Mallory, Frank Burr, and James Homer Wright. Pathological Technique. 7th ed. Philadelphia, PA: W.B. Saunders Company, 1918. 118-119.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining aorta.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Verhoeff-Van Gieson Elastic quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Elastic, Verhoeff Stain Kit: | Part 9116A/B | Individual Stain Solution | |
| Solution A: | Hematoxylin 5%, Alcoholic | 125/250 ml | Part 11623 |
| Solution B: | Ferric Chloride 10%, Aqueous | 125/250 ml | Part 10856 |
| Solution C: | Iodine, Lugol’s, Aqueous | 75/150 ml | Part 12092 |
| Solution D: | Sodium Thiosulfate 5%, Aqueous | 250/500 ml | Part 1389 |
| Solution E: | Van Gieson Stain | 250/500 ml | Part 1404 |
APPLICATION:
Newcomer Supply Elastic, Aorta Control Slides are for the positive histochemical staining of elastic fibers in artery.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- Prepare fresh Verhoeff Working Solution by combining in the exact order listed, mixing well after each addition. Save for Step #5.
-
- Solution A: Hematoxylin 5%, Alcoholic 20 ml
- Solution B: Ferric Chloride 10%, Aqueous 8 ml
- Solution C: Iodine, Lugol’s, Aqueous 8 ml
-
- Prepare fresh Ferric Chloride 2%, Aqueous Solution for Step #7.
-
- Solution B: Ferric Chloride 10%, Aqueous 10 ml
- Distilled water 40 ml
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in fresh Verhoeff Working Solution (Step #2) for 20 minutes.
-
- Discard solution after successful differentiation in Step #7.
-
- Rinse in several changes of tap water.
- Differentiate each slide individually in fresh Ferric Chloride 2%, Aqueous Solution (Step #3) with agitation; approximately 20 dips.
- Check differentiation: rinse well in tap water and check microscopically for black elastic staining with gray background.
-
- If necessary, return to Ferric Chloride 2%, Aqueous Solution until desired elastic differentiation is achieved.
- See Procedure Notes #3 and #4.
-
- Wash well in tap water.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous for 1 minute.
- Wash well in running tap water for 5 minutes.
- Counterstain in Solution E: Van Gieson Stain for 3 to 5 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Elastic fibers/tissue/nuclei | Blue-black to black |
| Collagen | Red |
| Other tissue elements | Yellow |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If background is colorless, the section has been over-differentiated.
-
- Over-differentiated sections may be re-stained in Step #5 provided sections have not been treated with alcohol.
-
- Differentiation can vary between slides dependent upon the amount of elastic present in sections.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 167-169.
- Mallory, Frank Burr, and James Homer Wright. Pathological Technique. 7th ed. Philadelphia, PA: W.B. Saunders Company, 1918. 118-119.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Mayer Mucicarmine quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs. .
CONTROL SLIDE VALIDATION:
| With Mucin, Mayer Mucicarmine Stain Kit: | Part 9151A/B | Individual Stain Solution | |
| Solution A: | Ferric Chloride, Aqueous | 125/250 ml | Part 1409 |
| Solution B: | Hematoxylin 1%, Alcoholic | 125/250 ml | Part 1409 |
| Solution C: | Mucicarmine Stock Stain, Mayer | 125/125 ml | Part 1250 |
| Solution D: | Metanil Yellow Stain, Aqueous | 250/500 ml | Part 12235 |
APPLICATION:
Newcomer Supply Cryptococcus Control Slides are for the positive histochemical staining of fungal organisms. The morphology of the organism is consistent with Cryptococcus sp.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
-
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Prepare fresh Weigert Iron Hematoxylin Working Solution directly before use; combine and mix well:
-
- Solution A: Ferric Chloride, Aqueous 20 ml
- Solution B: Hematoxylin 1%, Alcoholic 20 ml
-
- Stain in Weigert Iron Hematoxylin Working Solution for 7 minutes.
- Rinse in running tap water for 10 minutes.
- Prepare fresh Mayer Mucicarmine Working Solution; combine and mix well:
-
- Solution C: Mucicarmine Stock Stain, Mayer 10 ml
- Tap Water (do not use distilled water) 30 ml
-
- Stain slides in fresh Mayer Mucicarmine Working Solution for 60 minutes or longer if a more intense stain is desired.
Microwave Modification: See Procedure Note #3.
-
-
-
- Place slides in a plastic Coplin jar (Part 5184) containing fresh Mayer Mucicarmine Working Solution and microwave at 70°C for 10 minutes.
-
-
-
- Rinse in several changes of tap water.
- Counterstain in Solution D: Metanil Yellow Stain, Aqueous for 30 seconds to 1 minute.
- Dehydrate quickly through 95% and 100% ethyl alcohols. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Capsule of Cryptococcus | Deep rose to red |
| Nuclei | Black |
| Other tissue elements | Yellow |
PROCEDURE NOTES:
-
- Drain staining slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th ed. Chicago: ASCP Press, 2020. 149-151.
- Luna, Lee G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: Blakiston Division, McGraw-Hill, 1968. 161-162.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 168-169.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Rhodanine quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Copper, Rhodanine Stain Kit: | Part 9113A | Individual Stain Solution | |
| Solution A: | Rhodanine Stock Stain 0.2%, Alcoholic | 50 ml | Part 10531 |
| Solution B: | Hematoxylin Stain, Mayer Modified | 250 ml | Part 1202 |
| Solution C: | Sodium Borate 0.5%, Aqueous | 500 ml | Part 13824 |
APPLICATION:
Newcomer Supply Copper, Animal Control Slides are for the positive histochemical detection of copper in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Prepare Working Rhodanine Solution; combine and mix well.
- Shake Solution A: Rhodanine Stock Stain 0.2%, Alcoholic well before each use.
- Solution A: Rhodanine Stock Stain 0.2%, Alcoholic 3 ml
- Distilled Water 47 ml
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Stain in Working Rhodanine Solution (Step #2) at 60°C for 1-2 hours or at 37oC for 18 hours.
Microwave Modification: See Procedure Note #3.
- Place slides in a plastic Coplin jar containing Working Rhodanine Solution and microwave for 6 minutes at 70°C.
- At the end of incubation (for both oven and microwave), to avoid unwanted slide precipitate, pour off warm Working Rhodanine Solution into a second Coplin jar; reserve and set aside.
- Rinse slides well in several changes of distilled water.
- Check positive control slide microscopically to determine adequate copper/reddish brown development.
- Return slides to reserved Working Rhodanine Solution if additional incubation is required.
- Prepare dilute Mayer Hematoxylin Stain Solution directly before use; combine and mix well:
- Solution B: Hematoxylin Stain, Mayer Modified 20 ml
- Distilled Water 20 ml
- Stain in dilute Mayer Hematoxylin Stain Solution for 10 minutes.
- Rinse in distilled water.
- Rinse in Solution C: Sodium Borate 0.5%, Aqueous; 2-3 quick dips.
- Rinse well in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Copper | Copper/reddish brown |
| Nuclei | Light blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 251.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 258-260.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 230.
- Modifications developed by Newcomer Supply.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine and positive iron staining animal spleen.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Müller-Mowry Colloidal Iron quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Colloidal Iron, Müller-Mowry Stain Kit: | Part 9110A | Individual Stain Solution | |
| Solution A: | Acetic Acid 12%, Aqueous | 1000 ml | |
| Solution B: | Colloidal Iron Stock | 125 ml | Part 10365 |
| Solution C: | Acetic Acid, Glacial, ACS | 50 ml | Part 10010 |
| Solution D: | Potassium Ferrocyanide 2%, Aqueous | 125 ml | |
| Solution E: | Hydrochloric Acid 2%, Aqueous | 125 ml | |
| Solution F: | Van Gieson Stain | 250 ml | Part 1404 |
APPLICATION:
Newcomer Supply Colloidal Iron, Multi-Tissue Control Slides, use a combination of tissue sources for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin) and stromal (mesenchymal) mucin in small intestine and ferric iron in spleen.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Acid clean glassware prior to use to avoid residual iron staining.
- See Procedure Note #1.
- Prepare Colloidal Iron Working Solution; combine and mix well.
- Solution B: Colloidal Iron Stock 20 ml
- Solution C: Acetic Acid, Glacial ACS 5 ml
- Distilled Water 15 ml
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #2 and #3.
- Place in Solution A: Acetic Acid 12%, Aqueous for 30 seconds.
- Drain Slides. Do not rinse.
- Place in Colloidal Iron Working Solution (Step #2) for 30 minutes.
- Rinse in three changes of Solution A: Acetic Acid 12%, Aqueous; 3 minutes each.
- Prepare fresh Ferrocyanide-Hydrochloric Acid Solution directly before use; combine and mix well.
- Solution D: Potassium Ferrocyanide 2%, Aqueous 20 ml
- Solution E: Hydrochloric Acid 2%, Aqueous 20 ml
- Place in Ferrocyanide-Hydrochloric Acid Solution for 15 minutes.
- Wash in running tap water for 1-5 minutes.
- Counterstain in Solution F: Van Gieson Stain for 3-5 minutes.
- Proceed directly to dehydration step without rinsing.
- Dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Blue |
| Stromal mucin | Blue |
| Ferric iron deposits | Bright blue |
| Collagen | Red |
| Muscle and cytoplasm | Yellow |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Nuclear Fast Red Stain, Kernechtrot (Part 1255) can be used as an alternative counterstain.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 175-176.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 151-153.
- Rekhtman, Natasha, and Justin Bishop. Quick Reference Handbook for Surgical Pathologists. Berlin: Springer, 2011. 69.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Müller-Mowry Colloidal Iron quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Colloidal Iron, Müller-Mowry Stain Kit: | Part 9110A | Individual Stain Solution | |
| Solution A: | Acetic Acid 12%, Aqueous | 1000 ml | |
| Solution B: | Colloidal Iron Stock | 125 ml | Part 10365 |
| Solution C: | Acetic Acid, Glacial, ACS | 50 ml | Part 10010 |
| Solution D: | Potassium Ferrocyanide 2%, Aqueous | 125 ml | |
| Solution E: | Hydrochloric Acid 2%, Aqueous | 125 ml | |
| Solution F: | Van Gieson Stain | 250 ml | Part 1404 |
APPLICATION:
Newcomer Supply Colloidal Iron Control Slides are for the positive histochemical staining of acid epithelial mucins (sialomucin, sulfomucin) and stromal (mesenchymal) mucin in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- Acid clean glassware prior to use to avoid residual iron staining.
- See Procedure Note #1.
- Prepare Colloidal Iron Working Solution; combine and mix well.
- Solution B: Colloidal Iron Stock 20 ml
- Solution C: Acetic Acid, Glacial ACS 5 ml
- Distilled Water 15 ml
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #2 and #3.
- Place in Solution A: Acetic Acid 12%, Aqueous for 30 seconds.
- Drain Slides. Do not rinse.
- Place in Colloidal Iron Working Solution (Step #3) for 30 minutes.
- Rinse in three changes of Solution A: Acetic Acid 12%, Aqueous; 3 minutes each.
- Prepare fresh Ferrocyanide-Hydrochloric Acid Solution directly before use; combine and mix well.
- Solution D: Potassium Ferrocyanide 2%, Aqueous 20 ml
- Solution E: Hydrochloric Acid 2%, Aqueous 20 ml
- Place in Ferrocyanide-Hydrochloric Acid Solution for 15 minutes.
- Wash in running tap water for 1-5 minutes.
- Counterstain in Solution F: Van Gieson Stain for 3-5 minutes.
- Proceed directly to dehydration step without rinsing.
- Dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Blue |
| Stromal mucin | Blue |
| Collagen | Red |
| Muscle and cytoplasm | Yellow |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Nuclear Fast Red Stain, Kernechtrot (Part 1255) can be used as an alternative counterstain.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 175-176.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 151-153.
- Rekhtman, Natasha, and Justin Bishop. Quick Reference Handbook for Surgical Pathologists. Berlin: Springer, 2011. 69.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining rat lung.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Steiner-Steiner Modified Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Steiner-Steiner Modified Silver Stain Kit: | Part 9171A | Individual Stain Solution | |
| Solution A: | Uranyl Nitrate 1%, Aqueous | 250 ml | Part 14036 |
| Solution B: | Silver Nitrate 1%, Aqueous | 250 ml | Part 13804 |
| Solution C: | Gum Mastic 2.5%, Alcoholic | 350 ml | Part 1145 |
| Ingredient D: | Hydroquinone, Powder | 5 grams | Part 12089 |
APPLICATION:
Newcomer Supply Cat Scratch Artificial Control Slides are for the positive histochemical staining and qualitative identification of proteins of Bartonella sp., the causative bacterial agent of Cat Scratch disease.
Bartonella henselae purchased from American Type Culture Collection is used to produce the positive control tissue.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Preheat Solution A: Uranyl Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #9.
- Preheat Solution B: Silver Nitrate 1%, Aqueous to 60°C in a water bath. Save for Step #11.
- Prepare Hydroquinone Solution; combine and mix well.
-
- Ingredient D: Hydroquinone, Powder 5 gm (or one rounded scoop with reusable mini sampling spoon)
- Distilled Water 25 ml
-
-
- Prepare fresh Reducing Solution by combining in order listed.
-
- Hydroquinone Solution (Step #5) 25 ml
- Solution C: Gum Mastic 2.5%, Alcoholic 15 ml
- Solution B: Silver Nitrate 1%, Aqueous 6 ml
- Solution will turn milky white after addition of Gum Mastic.
- Preheat solution in 45°C water bath. Save for Step #15.
-
- Do not preheat solutions if using Microwave Modifications.
- Prepare fresh Reducing Solution by combining in order listed.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Note #3
-
- Sensitize in preheated Solution A: Uranyl Nitrate 1%, Aqueous (Step #3) for 10 minutes in a 60°C water bath.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #4.
-
-
-
- Place slides in a plastic Coplin jar with Solution A: Uranyl Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
-
-
- Rinse well in several changes of distilled water.
- Place slides in preheated Solution B: Silver Nitrate 1%, Aqueous (Step #4) and incubate in a 60°C water bath for 15 minutes.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Solution B: Silver Nitrate 1%, Aqueous. Microwave at 70°C for 1 minute.
- Remove from microwave, cover and let sit for 1 minute.
-
-
-
- Rinse well in several changes of distilled water.
-
- Excessive rinsing may cause nucleus to pick up silver.
-
- Dip 5 times in two changes each of 95% and 100% ethyl alcohols.
- Place in Solution C: Gum Mastic 2.5%, Alcoholic for 3 minutes.
- Place slides in preheated Reducing Solution (Step #6) in 45°C water bath for 10-30 minutes with frequent agitation. Examine microscopically after 10 minutes of incubation.
-
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution; check every 2-5 minutes until desired results are achieved.
-
- Rinse well in several changes of distilled water.
Microwave Modification:
-
-
-
- Place slides in a plastic Coplin jar with Reducing Solution. Microwave at 70°C for 1 minute. Remove from microwave.
- Pipette solution twice with plastic pipette to evenly distribute heated solution.
- Cover and let sit for 1 minute.
- Check microscopically by dipping slide in 100% alcohol.
- Review for desired staining results.
- If necessary, return to warm solution, check every 1 minute until desired results are achieved.
-
-
-
- Directly dehydrate in two changes of 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Bartonella | Dark brown to black |
| Background | Golden brown |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Garvey, Winsome. “Some Favorite Silver Stains.” The Journal of Histotechnology 3 (1996): 269-278.
- Luna, Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 218-219.
- Steiner, Gabriel, and Grete Steiner. “New Simple Silver Stain for Demonstration of Bacteria, Spirochetes and Fungi in Sections of Paraffin Embedded Tissue Blocks.” Journal of Laboratory Clinical Medicine 29 (1944). 868-871.
- Swisher, Billie. “Modified Steiner Procedure for Microwave Staining of Spirochetes and Nonfilamentous Bacteria.” The Journal of Histotechnology4 (1987): 241-243.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining placenta.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Von Kossa Calcium quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Von Kossa Calcium Stain: | Individual Stain Solution |
| Silver Nitrate 5%, Aqueous | Part 13805 |
| Sodium Thiosulfate 5%, Aqueous | Part 1389 |
| Nuclear Fast Red Stain, Kernechtrot | Part 1255 |
APPLICATION:
Newcomer Supply Calcium Control Slides are for the positive histochemical staining of calcium or calcium salts in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to you laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Place slides in Silver Nitrate 5%, Aqueous according to the following timings and conditions.
- Direct sunlight or ultraviolet light for 10-30 minutes.
- In front of a 60-100 watt light bulb for 1 hour or longer.
- See Procedure Note #5.
- Check slides periodically and remove from light source when control slide shows black-brown deposits macroscopically.
- Rinse well in several changes of distilled water.
- Place slides in Sodium Thiosulfate 5%, Aqueous for 2 minutes.
- Rinse well in several changes of distilled water.
- Counterstain in Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #6.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol; 10 dips each. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Calcium salts | Black to brown/black |
| Nuclei | Red |
| Cytoplasm | Light pink |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Direct sunlight is the preferred method. If procedure is carried out in minimal sunlight increased incubation time will be necessary.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 269-270.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 226-227.
- Modifications developed by Newcomer Supply Laboratory.
PRODUCT SPECIFICATIONS:
Tissue: Positive staining animal organ.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Grocott Methenamine Silver quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fungus, Grocott Methenamine Silver (GMS) Stain Kit: | Part 9121A/B | Individual Stain Solution | |
| Solution A: | Chromic Acid 5%, Aqueous | 250/500 ml | Part 10341 |
| Solution B: | Sodium Bisulfite 1%, Aqueous | 250/500 ml | Part 13821 |
| Solution C: | Silver Nitrate | 125/250 ml | Part 1142 |
| Solution D: | Methenamine Borate | 125/250 ml | Part 1142 |
| Solution E: | Gold Chloride 0.1%, Aqueous | 250/500 ml | Part 11285 |
| Solution F: | Sodium Thiosulfate 2%, Aqueous | 250/500 ml | Part 13888 |
| Solution G: | Light Green SF Yellowish Stain 0.2%, Aqueous | 250/500 ml | Part 12202 |
APPLICATION:
Newcomer Supply Blastomyces, Animal Control Slides are for the positive histochemical staining of fungal organisms. The morphology of the organisms is consistent with Blastomyces sp.
PRESTAINING PREPARATION:
-
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
-
- See Procedure Notes #1 and #2.
-
- Prepare Silver-Methenamine Working Solution and mix well.
-
- Solution C: Silver Nitrate 20 ml
- Solution D: Methenamine Borate 20 ml
-
- Preheat Silver-Methenamine Working Solution to 45°C-60°C in a water bath approximately 20 to 30 minutes before use.
-
- See Procedure Note #3.
- Do not preheat if using Microwave Modification; Step 11.
-
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #4 and #5.
-
- Oxidize in Solution A: Chromic Acid 5%, Aqueous for 1 hour.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
Microwave Modification: See Procedure Note #6
-
-
-
- Microwave Solution A: Chromic Acid 5%, Aqueous without slides in a plastic Coplin jar (Part 5184) for 1 minute at 60°C. Add slides to heated Solution A and oxidize for 10 minutes.
-
- Wash well in running tap water; rinse in distilled water.
- Place in Solution B: Sodium Bisulfite 1%, Aqueous for 1 minute.
- Wash in running tap water for 5 minutes; rinse well in distilled water.
- Incubate slides in preheated Silver-Methenamine Working Solution (Step #4) at 45°C-60°C or at room temperature, for 12-18 minutes until sections appear paper-bag brown.
-
-
- Periodically remove control, rinse in warm distilled water, check microscopically for adequate silver impregnation. Fungi should be dark brown.
- If organisms are not sufficiently dark, return slides to warm silver solution. Recheck at 2-3 minute intervals until desired intensity is achieved.
- Staining at room temperature will require longer incubation.
Microwave Modification:
- Incubate slides in a plastic Coplin jar containing Silver-Methenamine Working Solution and microwave for 5 minutes at 45°C.
- Check microscopically for adequate development.
- If additional incubation is required, return slides to warm silver solution. Recheck at 3-5 minute intervals.
-
- Rinse in three to four changes of distilled water.
- Do not use tap water at this step.
- Tone in Solution E: Gold Chloride 0.1%, Aqueous until sections turn gray; 10-30 seconds.
- Rinse well in distilled water.
- Remove unreduced silver in Solution F: Sodium Thiosulfate 2%, Aqueous for 2 minutes.
- Wash in running tap water for 5 minutes; rinse in distilled water.
- Counterstain in Solution G: Light Green SF Yellowish Stain 0.2%, Aqueous for 2 minutes.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
-
RESULTS:
| Blastomyces | Crisp black cell walls & visible internal structures |
| Mucin | Taupe to dark gray |
| Background | Green |
PROCEDURE NOTES:
-
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Maintain solution between 45°C-60°C to minimize precipitate.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, follow manufacturer’s recommendation for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Cappellano. Histotechnology: A Self-Instructional Text. 5th ed. Chicago: ASCP Press, 2020. 221-226.
- Grocott, R G, “A Stain for Fungi in Tissue Sections and Smears using Gomori Methenamine Silver Nitrate Technic”. American Journal of Clinical Pathology 25 (1955): 975-979.
- Koski, John. “Silver Methenamine Borate (SMB): Cost Reduction with Technical Improvement in Silver Nitrate-Gold Chloride Impregnations.” The Journal of Histotechnology 3 (1981): 115-119.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 245-246.
- Modifications developed by Newcomer Supply Laboratory.




