Phosphotungstic Acid Hematoxylin (PTAH) Stain Kit
![]()
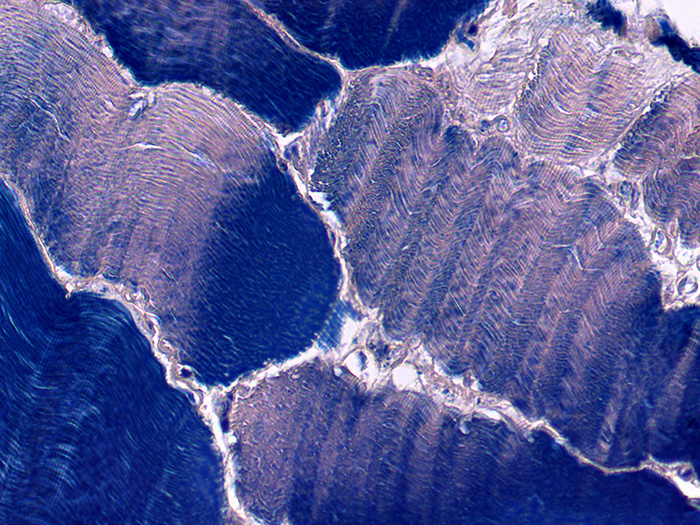
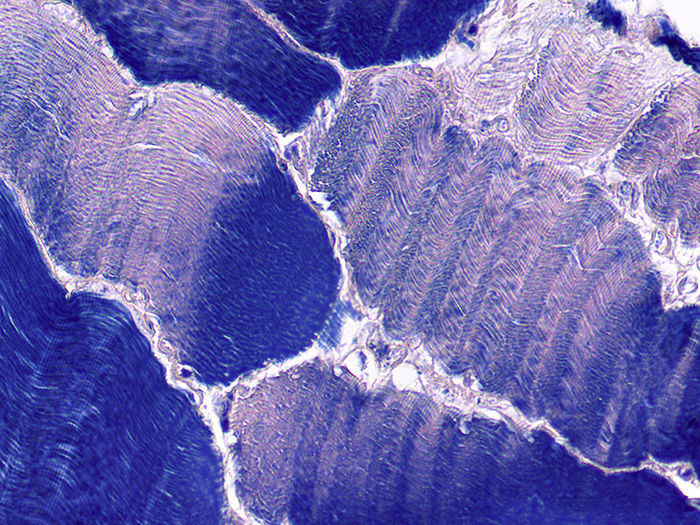
Used for the demonstration of collagen, muscle striations and central nervous system (CNS) structures. The Zenker fixative used in this procedure is modified with zinc chloride and is mercury free.
PHOSPHOTUNGSTIC ACID HEMATOXYLIN (PTAH) STAIN KIT INCLUDES:
| Part 9111A | ||
| Solution A: | Zenker Fixative, Modified, Zinc Chloride | 250ml |
| Solution B: | Acetic Acid, Glacial, ACS | 25ml |
| Solution C: | Potassium Permanganate 0.25%, Aqueous | 250ml |
| Solution D: | Oxalic Acid 5%, Aqueous | 250ml |
| Solution E: | Phosphotungstic Acid Hematoxylin (PTAH) Stain | 250ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Coplin Jar, Plastic | Part 5184 (for microwave modification) |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
The Newcomer Supply Phosphotungstic Acid Hematoxylin (PTAH) Stain Kit procedure, with included microwave modification, is used for the demonstration of collagen, muscle striations and central nervous system (CNS) structures. The Zenker fixative used in this procedure is modified with zinc chloride and does not contain mercury.
METHOD:
Fixation: 10% Phosphate Buffered Formalin (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- Prepare Zenker Fixative Working Solution; combine and mix well.
Solution A: Zenker Fixative, Modified, Zinc Chloride 38 ml
Solution B: Acetic Acid, Glacial, ACS 2 ml
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Fix in Zenker Fixative Working Solution (Step #2) at 56°C; 3 hours.
Microwave Modification: See Procedure Note #3.
-
-
- Place slides in a plastic Coplin jar containing Zenker Fixative Working Solution and microwave for 5 minutes at 60°C.
-
- Wash well in three changes of tap water; rinse in distilled water.
- Place in Solution C: Potassium Permanganate 0.25%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Solution D: Oxalic Acid 5%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Stain in Solution E: PTAH Stain for 12-24 hours at room temperature, or 2 hours at 56°C.
-
- See Procedure Note #4.
-
Microwave Modification:
-
-
- Place slides in a plastic Coplin jar containing Solution E: PTAH Stain and microwave for 7 minutes at 70°C.
-
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each, coverslip with compatible mounting medium.
-
-
- Dehydrate quickly, alcohol may extract stain.
-
RESULTS:
| Collagen, cartilage, elastic fibers | Deep reddish brown |
| Muscle striations, fibrin, keratin | Dark blue |
| Glia fibers | Dark blue |
| Myelin | Lighter blue |
| Neurons | Salmon/Pink |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- The PTAH Stain formulation is twice as strong as the original Mallory formulation; adjust staining time according to preference of intensity. Suggested staining time at 37°C is 18 hours.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008.130-131.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015.. 178-180, 201-202.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 193-194.
- Modifications developed by Newcomer Supply Laboratory.