Periodic Acid Schiff (PAS) Glycogen
PRODUCT SPECIFICATIONS:
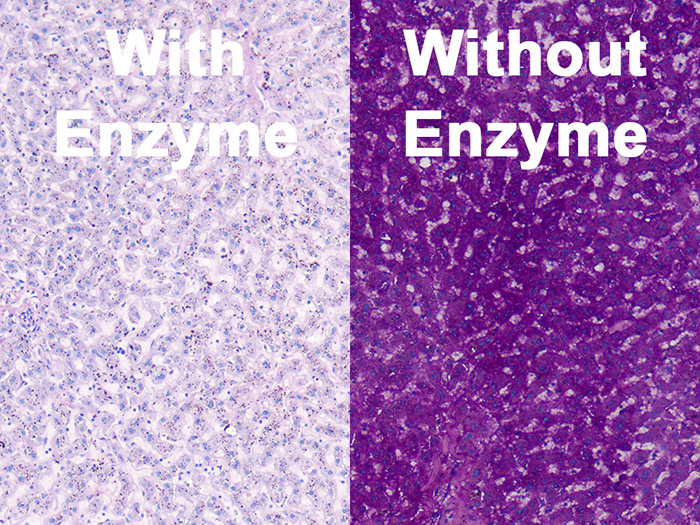
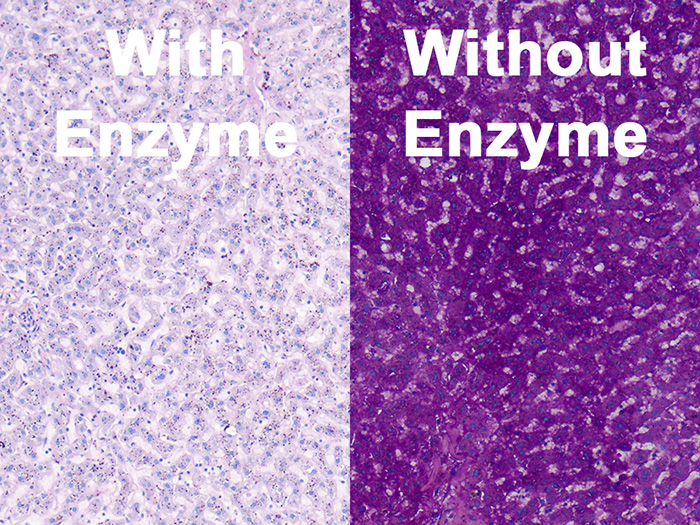
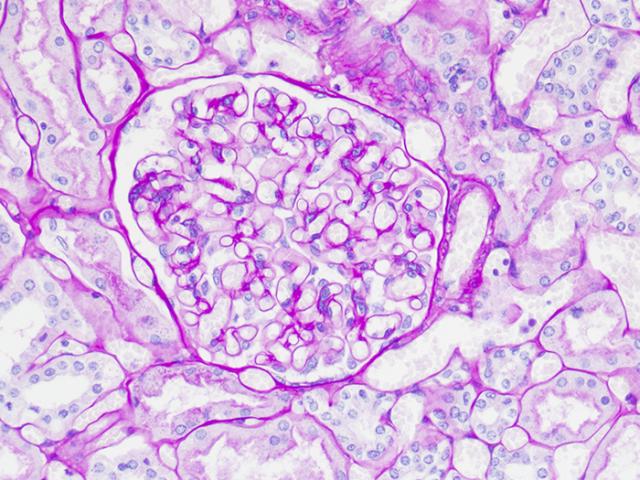
Tissue: Positive staining liver.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: PAS quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Periodic Acid Schiff (PAS) Stain Kit: | Part 9162A/B | Individual Stain Solution |
| Solution A: Periodic Acid 0.5%, Aqueous | 250/500 ml | Part 13308 |
| Solution B: Schiff Reagent, McManus | 250/500 ml | Part 1371 |
| Solution C: Hematoxylin Stain, Harris | 250/500 ml | Part 12013 |
| Solution D: Acid Alcohol 1% | 250/500 ml | Part 10011 |
| Solution E: Lithium Carbonate, Saturated Aqueous | 250/500 ml | Part 12215 |
| Alpha Amylase 1%, Aqueous (for glycogen digestion) | Part 1905 | |
| Coplin Jar, Plastic (for glycogen digestion microwave modification) | Part 5184 | |
APPLICATION:
Newcomer Supply Periodic Acid Schiff (PAS) Glycogen Control Slides are for the positive histochemical staining of glycogen in tissue sections and can also be utilized as control slides for glycogen digestion steps.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Proceed to Step #5 if not running Digestion
- Digestion Step: See Procedure Note #3.
- Two control slides and two patient slides are needed.
- Label one control slide and one patient slide “with”.
- Label the other control slide and patient slide “without”.
- Place slides labeled “without” in separate Coplin jar of distilled water; hold for Step #5.
- Apply Alpha Amylase 1%, Aqueous (Part 1905) to slides labeled “with” for 30 minutes at room temperature.
- Proceed to Step #5.
- Microwave Modification: See Procedure Note #4.
- Follow Steps #3a through #3d.
- Place slides labeled “with” in a plastic Coplin jar containing Alpha Amylase 1%, Aqueous (Part 1905) and microwave for 1 minute at 37°C. Let sit in warm solution for an additional minute.
- Combine all slides for remaining steps; wash in running tap water for 1 minute; rinse in distilled water.
- Place in Solution A: Periodic Acid 0.5%, Aqueous for 10 minutes.
- Wash in three changes of tap water; rinse in distilled water.
- Place in Solution B: Schiff Reagent, McManus for 20 minutes.
- Wash in lukewarm tap water for 5 minutes.
- Stain with Solution C: Hematoxylin Stain, Harris, 1 to 5 minutes, depending on preference of nuclear stain intensity.
- Wash in tap water for 2-3 minutes.
- Differentiate in Solution D: Acid Alcohol 1%; 1-2 quick dips.
- Wash in tap water for 1 minute.
- Blue in Solution E: Lithium Carbonate, Saturated Aqueous; 3-4 dips.
- Wash in several changes of tap water; rinse in distilled water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Glycogen digestion | Absence of magenta |
| Glycogen | Magenta |
| Acid & neutral epithelial mucin | Magenta |
| Nuclei | Blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Slides labeled “with” will be treated with amylase digestion, slides labeled “without” will not be treated for digestion.
- The suggested microwave procedure has been tested at Newcomer Supply. This procedure is a guideline and techniques should be developed for use in your laboratory.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 168-171, 180.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.137-141.
- Sheehan, Dezna C., and Barbara B.Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 164-168.
- Modifications developed by Newcomer Supply Laboratory.