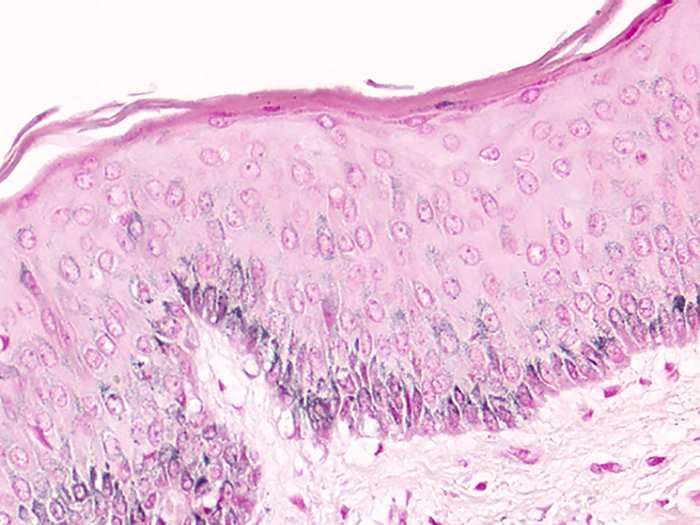
Melanin
|
Validation Stain: Fontana Masson
Other Applicable Stains: Schmorl Melanin
|
PRODUCT SPECIFICATIONS:
Tissue: Positive staining skin.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Fontana Masson quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Fontana Masson Stain Kit: | Part 9105A | Individual Stain Solution | |
| Solution A: | Silver Nitrate 10%, Aqueous | 250 ml | Part 13806 |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 250 ml | Part 1006 |
| Solution C: | Gold Chloride 0.2%, Aqueous | 250 ml | Part 11286 |
| Solution D: | Sodium Thiosulfate 5%, Aqueous | 250 ml | Part 1389 |
| Solution E: | Nuclear Fast Red Stain, Kernechtrot | 250 ml | Part 1255 |
APPLICATION:
Newcomer Supply Melanin Control Slides are for the positive histochemical staining of melanin in tissue sections.
PRESTAINING PREPARATION:
- Heat dry sections in oven according to your laboratory protocol.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Prepare Fontana Silver Working Solution (diamine silver) in an acid cleaned Erlenmeyer flask:
- Solution A: Silver Nitrate 10%, Aqueous; 25 ml
- Add Solution B: Ammonium Hydroxide 28-30%, ACS drop by drop, mix with swirling motion until solution clouds, then clears. Use caution to not add too much Solution B: Ammonium Hydroxide 28-30%, ACS.
- Add more Solution A: Silver Nitrate 10%, Aqueous drop by drop until clear solution becomes slightly cloudy.
- Let solution stand for 2-4 hours before use.
- For use; after standing, filter the solution. Combine 20 ml of this filtered diamine silver solution with 40 ml of distilled water; 60 ml total.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Immerse slides in the Fontana Silver Working Solution (Step #3) in a 45°C to 60°C water bath for 1 hour.
- Check slides microscopically; remove control, rinse in warm distilled water. Confirm that reaction is complete when granules are dark brown and background is colorless.
- Return to heated Fontana Silver Working Solution for longer incubation if indicated.
- Rinse well in three changes of distilled water.
- Immerse in Solution C: Gold Chloride 0.2%, Aqueous; 10 minutes.
- Rinse well in distilled water.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous; 5 minutes.
- Rinse well in distilled water.
- Counterstain in Solution E: Nuclear Fast Red Stain, Kernechtrot for 5 minutes.
- Shake solution well before use; do not filter.
- Rinse well in distilled water.
- See Procedure Note #5.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Melanin | Black |
| Nuclei | Pink-red |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with any silver solution to prevent precipitation of silver salts. No metals of any kind should be in contact with any silver solution. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Wash well after Nuclear Fast Red Stain, Kernechtrot to avoid cloudiness in dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Lee G. Histopathologic Methods and Color Atlas of Special Stains and Tissue Artifacts. Gaitheresburg, MD: American Histolabs, 1992. 286-287.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 276-277.
- Modifications developed by Newcomer Supply Laboratory.