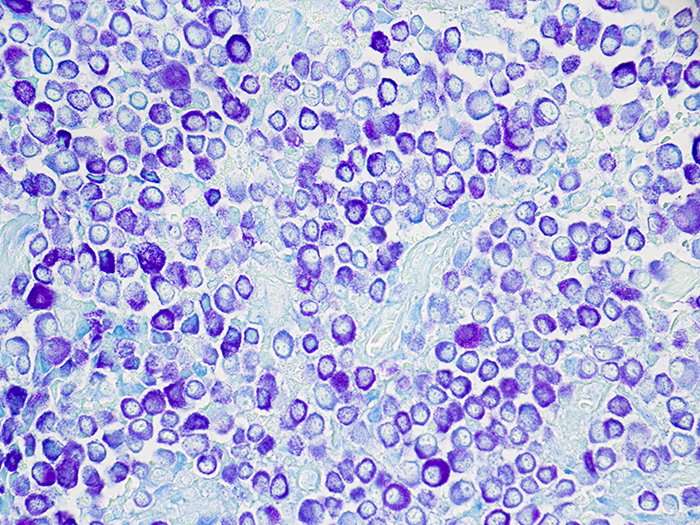
Mast Cell, Toluidine Blue Stain
Reagents for this procedure are sold as individual stain solutions and are available for purchase under separate part numbers with storage requirements and expiration date designated per bottle.
Technical Memo 1: Toluidine Blue Stain for Mast Cells
SOLUTION:
| 250 ml | 500 ml | 1 Gallon | |
| Toluidine Blue Stain 0.1%, Aqueous | Part 14027A | Part 14027B | Part 14027D |
Additionally Needed:
| Mast Cell Control Slides OR Mast Cell, Animal Control Slides |
Part 4410 OR Part 4412 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Toluidine Blue Stain for Mast Cells is for the demonstration of mast cells, characterized as cells filled with basophilic granules, associated with inflammation and allergic reactions, which stain metachromatically with toluidine blue.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 5 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Toluidine Blue Stain 0.1%, Aqueous for 10 minutes.
- Rinse well in distilled water.
- Dehydrate quickly through two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- See Procedure Note #3.
RESULTS:
| Mast cells | Deep blue-violet |
| Background | Blue |
PROCEDURE NOTES:
- Drain staining rack/slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during staining procedure.
- Metachromasia of mast cell granules is stable and staining will be maintained during dehydration steps.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Broome, Michelle and Beth Villarreal. “Differential Staining of Mast Cells with Toluidine Blue”. The Journal of Histotechnology 35.1 (2012): 27-30.
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009.188.
- Modifications developed by Newcomer Supply Laboratory.
Technical Memo 2: Toluidine Blue Stain for Mohs Technique
SOLUTION:
| 250 ml | 500 ml | 1 Gallon | |
| Toluidine Blue Stain 0.1%, Aqueous | Part 14027A | Part 14027B | Part 14027D |
Additionally Needed:
| Alcohol, Ethyl Denatured, 70% | Part 10844 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Xylene, ACS | Part 1445 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Toluidine Blue Stain for Mohs Technique provides a rapid staining method for Mohs micrographic surgery (MMS), useful when evaluating frozen skin samples for basal cell carcinoma (BCC). Toluidine Blue imparts an identifiable staining pattern if BCC is present that will highlight islands of blue staining basal cell carcinoma and metachromatically stain surrounding mucopolysaccharides/stroma pink.
METHOD:
Fixation: 70% Ethyl Alcohol (Part 10844)
Technique: Frozen sections cut at 5-7 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below.
STAINING PROCEDURE:
- Fix tissue sections in 70% Ethyl Alcohol for 30-60 seconds.
- See Procedure Note #1.
- Wash well in distilled water to remove excess fixative.
- Stain slides in Toluidine Blue Stain 0.1%, Aqueous for 30-60 seconds, depending on preference of stain intensity.
- Wash gently in distilled water to remove excess stain.
- Dehydrate quickly through one change of 95% ethyl alcohol; 1 quick dip and then two changes 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- See Procedure Note #2.
RESULTS:
| Islands of basal cell carcinoma | Deep blue to purple |
| Surrounding mucopolysaccharides/stroma | Pink to magenta |
| Background | Blue |
| Nuclei | Dark blue |
PROCEDURE NOTES:
- Section thickness and fixation timing may affect staining quality.
- Alcohol will work as a differentiator. Proceed quickly through dehydration steps to maintain Toluidine Blue stain.
- If using a xylene substitute, closely follow the manufacturer’s recommendation for clearing step.
REFERENCES:
- Arnon, Ofer, Ronald Rapini, Adam Mamelak, and Leonard Goldberg. “Mohs Micrographic Surgery: Current Techniques.” IMAJ 12 (2010): 431-35.
- Gross, Kenneth G. Mohs Surgery: Fundamentals and Techniques. St. Louis: Mosby, 1999. 125-138.
- Modifications developed by Newcomer Supply Laboratory.