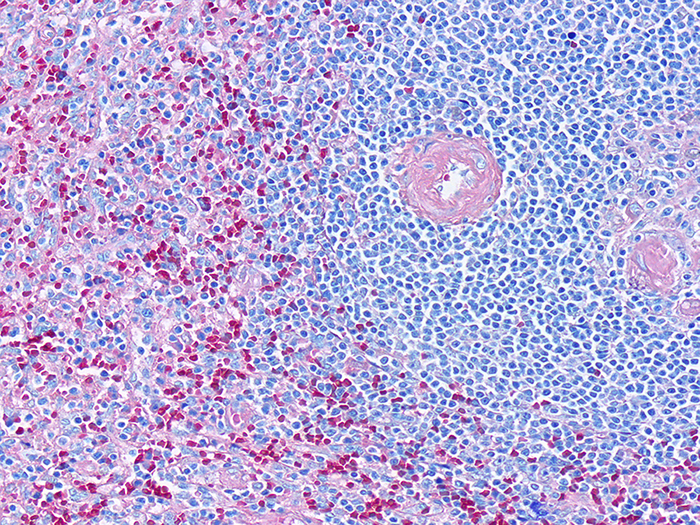
Hematopoietic
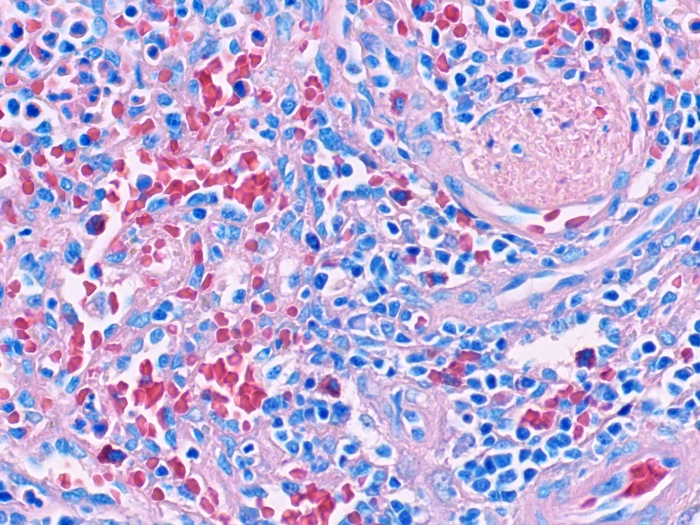
Stain solution groups are sold as individual products under separate part numbers with storage requirements and expiration date designated per bottle.
Giemsa, May-Grunwald
Click “View Product” to display stains & solutions for this procedure. Reagents are sold under separate part numbers with storage requirements and expiration date designated per bottle.
Giemsa, Wolbach Stain
Click “View Product” to display stains & solutions for this procedure. Reagents are sold under separate part numbers with storage requirements and expiration date designated per bottle.
Giemsa, Wright Stain, Modified for Tissue Sections
Click “View Product” to display stains & solutions for this procedure. Reagents are sold under separate part numbers with storage requirements and expiration date designated per bottle.
Wright Stain, Buffered
Click “View Product” to display stains & solutions for this procedure. Reagents are sold under separate part numbers with storage requirements and expiration date designated per bottle.