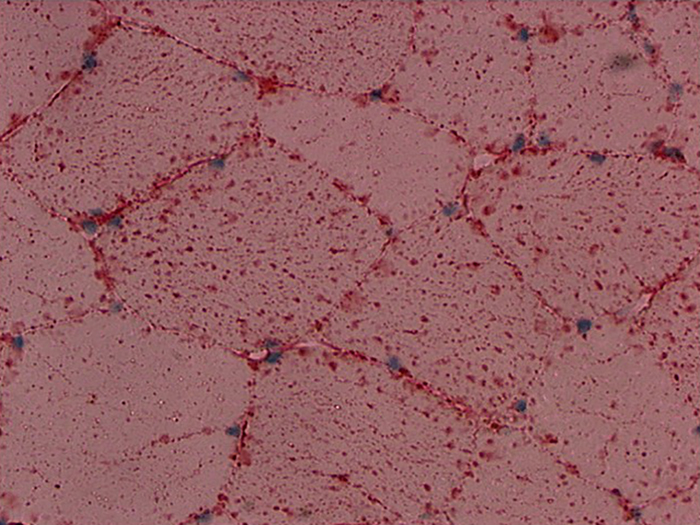
Fat, Oil Red O, Propylene Glycol Stain Kit
Used for identification of fat/lipid in frozen sections.
FAT, OIL RED O, PROPYLENE GLYCOL STAIN KIT INCLUDES:
| Part 9119A | ||
| Solution A: | Propylene Glycol 100%, ACS | 250 ml |
| Solution B: | Oil Red O Stain, Propylene Glycol | 250 ml |
| Solution C: | Propylene Glycol 85%, Aqueous | 250 ml |
| Solution D: | Hematoxylin Stain, Mayer Modified | 250 ml |
Individual stain solutions may be available for purchase under separate part numbers.
Additionally Needed:
| Formalin 10%, Phosphate Buffered | Part 1090 |
| Lithium Carbonate, Saturated Aqueous OR Scott Tap Water Substitute |
Part 12215 OR Part 1380 |
| Mount-Quick Aqueous | Part 6271A |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Fat, Oil Red O, Propylene Glycol Stain Kit procedure, classified as a physical staining method, is used for identification of fat/lipid in frozen sections.
METHOD:
Fixation: Fresh tissue or formalin fixed unprocessed tissue
-
-
- See Procedure Note #1.
-
Technique: Frozen sections cut at 8-10 microns on adhesive slides
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
STAINING PROCEDURE:
-
- Fix frozen section slides in Formalin 10%, Phosphate Buffered (Part 1090) for 1 minute.
-
- See Procedure Note #2.
-
- Rinse sections carefully in two changes of distilled water.
- Blot off excess water and place slides in Solution A: Propylene Glycol 100%, ACS with agitation for 2-5 minutes.
- Place slides directly into Solution B: Oil Red O Stain, Propylene Glycol for 1 hour. Agitate occasionally or place Coplin jar on rotator/shaker with continuous gentle agitation.
-
- See Procedure Notes #3 and #4.
-
- Differentiate in Solution C: Propylene Glycol 85%, Aqueous with agitation for a minimum of 3 minutes.
- Rinse gently in two changes of distilled water.
- Counterstain with Solution D: Hematoxylin Stain, Mayer Modified, for 2-3 minutes.
- Wash gently in several changes of tap water.
- Optional: Blue slides in Lithium Carbonate, Saturated Aqueous (Part 12215) or Scott Tap Water Substitute (Part 1380) for 10 dips.
- Wash gently in several changes of tap water.
- Blot excess water from slide; coverslip with Mount-Quick Aqueous (Part 6271A) mounting medium.
-
- See Procedure Note #5.
-
- Fix frozen section slides in Formalin 10%, Phosphate Buffered (Part 1090) for 1 minute.
RESULTS:
| Fat: | Bright red |
| Nuclei: | Blue to dark blue |
PROCEDURE NOTES:
-
- To freeze formalin fixed unprocessed tissue:
-
- Place fixed specimen in tissue cassette, wash in running water for 5 minutes.
- Remove tissue and blot excess water from tissue.
- Freeze tissue (fresh or formalin fixed) according to your laboratory protocol.
-
- Frozen formalin fixed tissue does not require additional fixation.
- To decrease staining time; preheat Solution B: Oil Red O Stain, Propylene Glycol in a 60oC oven and incubate for 7-10 minutes.
- If filmy precipitate forms on Solution B: Oil Red O Stain, Propylene Glycol, skim the surface with filter paper before use.
- Use minimal pressure when applying coverslip or fat/lipid staining may be disturbed. To remove trapped air bubbles or to recoverslip;
-
- Soak slide in warm water until coverslip is easily removed.
- Blot excess water from slide.
- Remount with new coverslip and Mount-Quick Aqueous mounting medium.
-
- To freeze formalin fixed unprocessed tissue:
REFERENCES:
-
- Prophet, Edna B., Bob Mills, Jacquelyn Arrington, and Leslie Sobin. Laboratory Methods in Histotechnology. Washington, D.C.: American Registry of Pat 1992.178.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 205.
- Modifications developed by Newcomer Supply Laboratory.