Bielschowsky, Lester-King Modified Stain Kit
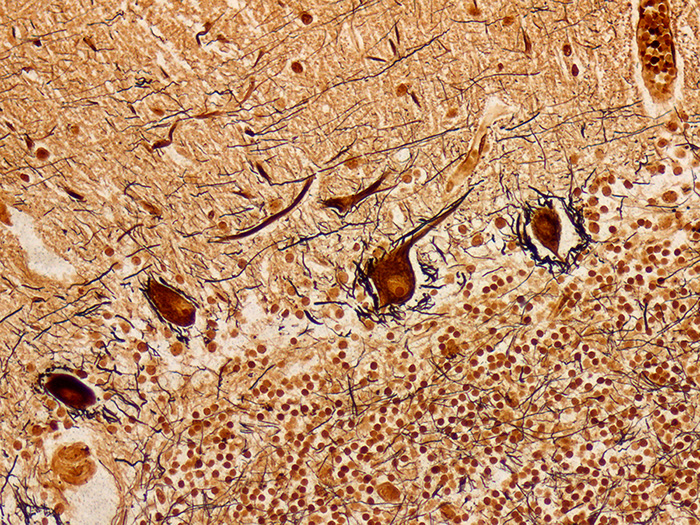
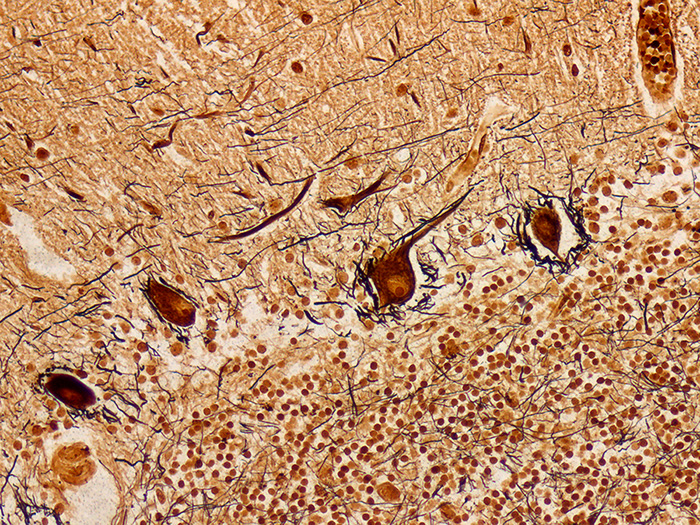
Stains nerve fibers, neurofibrils/tangles, senile plaques and axons. Used in the diagnosis of Alzheimer’s disease and other neurological disorders.
BIELSCHOWSKY, LESTER-KING MODIFIED STAIN KIT INCLUDES:
| Part 9154A | ||
| Solution A: | Silver Nitrate 20%, Aqueous | 250 ml |
| Solution B: | Ammonium Hydroxide 28-30%, ACS | 100 ml |
| Solution C: | Developer | 25 ml |
| Solution D: | Sodium Thiosulfate 5%, Aqueous | 250 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed with this kit are two complimentary unstained positive control slides to be used for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers .
Additionally Needed:
| Hydrochloric Acid 5%, Aqueous | Part 12086 (for acid cleaning glassware) |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply Bielschowsky, Lester King Modified Stain Kit procedure is used to demonstrate nerve fibers, neurofibrils/tangles, senile plaques and axons. This stain can be instrumental in the diagnosis of Alzheimer’s disease and other neurological disorders.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 8 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
- If necessary, heat dry tissue sections/slides in oven.
- All glassware/plasticware must be acid cleaned prior to use.
- See Procedure Notes #1 and #2.
- Preheat Coplin jar of Solution A: Silver Nitrate 20%, Aqueous in water bath to 37°C.
- Reserve two acid cleaned Coplin jars filled with distilled water.
- Save for slide rinsing/holding in Steps #7 and #10.
STAINING PROCEDURE:
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #3 and #4.
- Place in preheated Solution A: Silver Nitrate 20%, Aqueous (Step #3) for 15 minutes.
- See Procedure Note #5.
- Remove slides from Solution A: Silver Nitrate 20%, Aqueous and hold in 1st reserved Coplin jar of distilled water.
- Save Silver Nitrate 20%, Aqueous; pour into acid cleaned Erlenmeyer flask for Step #8.
- Add Solution B: Ammonium Hydroxide 28-30%, ACS drop by drop into the saved Silver Nitrate 20%, Aqueous, swirling until precipitate disappears.
- Solution will initially be brown in color and then clear. Do not add excess ammonium hydroxide.
- Pour Ammoniacal Silver Solution back into acid cleaned Coplin jar.
- Return held slides to Ammoniacal Silver Solution in 37°C water bath for 10 minutes.
- Remove slides and hold in 2nd reserved Coplin jar of distilled water.
- Save Ammoniacal Silver Solution for Step #11.
- Add 1 drop of Solution C: Developer to the saved Ammoniacal Silver Solution with swirling motion.
- Return slides to Ammoniacal Silver Solution with added Developer, in 37°C water bath for 5-15 minutes; average time of 6 minutes.
- Check slides microscopically at 3 minutes for development of neurons to dark brown.
- Follow with checks at 1 minute intervals.
- Rinse thoroughly in distilled water for 5 minutes.
- Place in Solution D: Sodium Thiosulfate 5%, Aqueous; 5 minutes.
- Rinse thoroughly in tap water.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Senile plaques, neurofibrils/tangles | Dark brown to black |
| Neurons | Dark brown |
| White and gray matter | Yellowish brown |
| Nerve fibers, axons | Brown to black |
PROCEDURE NOTES:
- Acid clean all glassware/plasticware (Part 12086) and rinse thoroughly in several changes of distilled water.
- Plastic (Part 5500), plastic-tipped or paraffin coated metal forceps must be used with silver solutions to prevent precipitation of silver salts. No metals of any kind should come in contact with silver solutions. Only glass thermometers should be used.
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Eight slides per 40 ml of Solution A: Silver Nitrate 20%, Aqueous is recommended for proper silver development.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 196-199.
- King, Lester. “The Impregnation of Neurofibrils”. Yale Journal of Biology and Medicine 14.1 (1941). 59-68.
- Modifications developed by Newcomer Supply Laboratory.