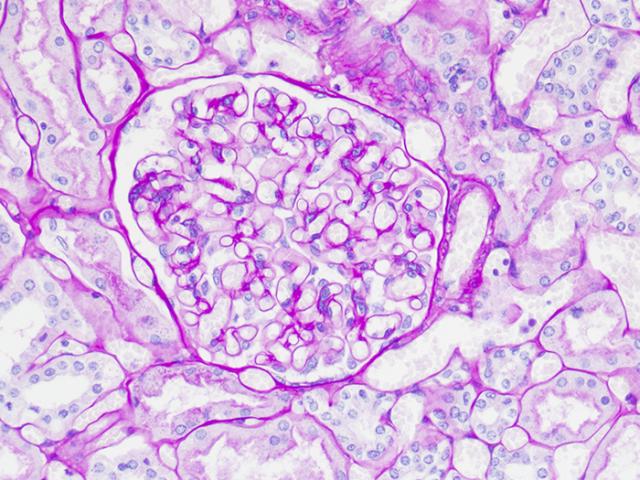
Alcian Blue/PAS
|
Validation Stain: Alcian Blue/PAS
|
PRODUCT SPECIFICATIONS:
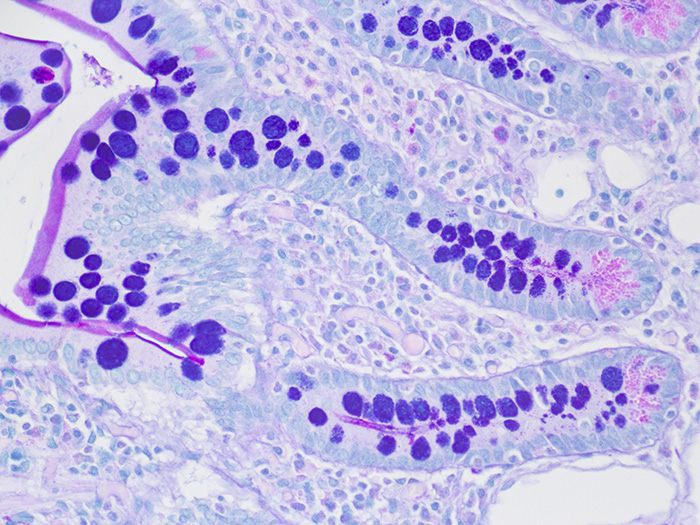
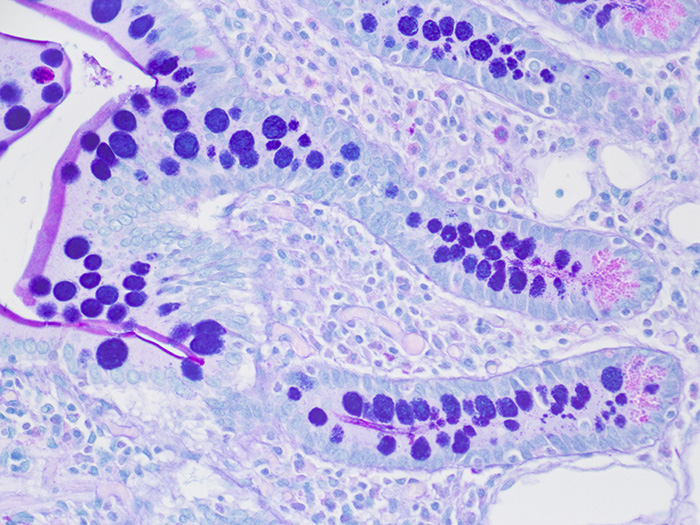
Tissue: Positive staining small intestine.
Fixation: Formalin 10%, Phosphate Buffered (Part 1090).
Section/Glass: Paraffin sections cut at 4 microns on Superfrost™ Plus slides.
Quality Control Stain: Alcian Blue/PAS quality control stained slide(s) included.
Reactivity: Guaranteed product specific reactivity for one year from date of receipt. Revalidate after one year to verify continued reactivity.
Storage: 15-30°C in a light deprived and humidity controlled environment.
Intended Use: To verify histological techniques and reagent reactivity.
Before using unstained control slides, review the enclosed stained slide(s) to ensure that this tissue source is acceptable for testing needs.
CONTROL SLIDE VALIDATION:
| With Alcian Blue/PAS Stain Kit: | Part 91022A/B | Individual Stain Solution | |
| Solution A: | Acetic Acid 3%, Aqueous | 250/500 ml | Part 10017 |
| Solution B: | Alcian Blue Stain 1%, pH 2.5 Aqueous | 250/500 ml | Part 1003 |
| Solution C: | Periodic Acid 0.5%, Aqueous | 250/500 ml | Part 13308 |
| Solution D: | Schiff Reagent, McManus | 250/500 ml | Part 1371 |
| Solution E: | Hematoxylin Stain, Mayer Modified | 250/500 ml | Part 1202 |
APPLICATION:
Newcomer Supply Alcian Blue/PAS Control Slides are for the positive histochemical staining and differentiation of acidic epithelial mucins (sialomucin, sulfomucin), stromal (mesenchymal) mucin, neutral mucins and glycogen.
NEWCOMER SUPPLY VALIDATION PROCEDURE:
- Heat dry sections in oven according to your laboratory protocol.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
- See Procedure Notes #1 and #2.
- Place slides in Solution A: Acetic Acid 3%, Aqueous for 3 minutes.
- Place slides directly into Solution B: Alcian Blue Stain 1%, pH 2.5 Aqueous for 15 minutes.
- Wash slides in gently running tap water for 1-2 minutes; rinse in distilled water.
- Place in Solution C: Periodic Acid 0.5%, Aqueous for 5 minutes.
- Wash in running tap water for 1-2 minutes; rinse in distilled water.
- Place in Solution D: Schiff Reagent, McManus for 10 minutes.
- Wash in lukewarm tap water for 5-10 minutes.
- Stain lightly in Solution E: Hematoxylin Stain, Mayer Modified for 1 minute.
- Rinse in running tap water for 1-2 minutes.
- Dehydrate in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
RESULTS:
| Acid epithelial mucins | Violet |
| Neutral epithelial mucin | Magenta |
| Glycogen | Magenta |
| Stromal (mesenchymal) mucin | Blue |
| Nuclei | Pale blue |
PROCEDURE NOTES:
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
- Bancroft, John D., and Marilyn Gamble. Theory and Practice of Histological Techniques. 6th ed. Oxford: Churchill Livingstone Elsevier, 2008. 173-174.
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 150-151
- Modifications developed by Newcomer Supply Laboratory.