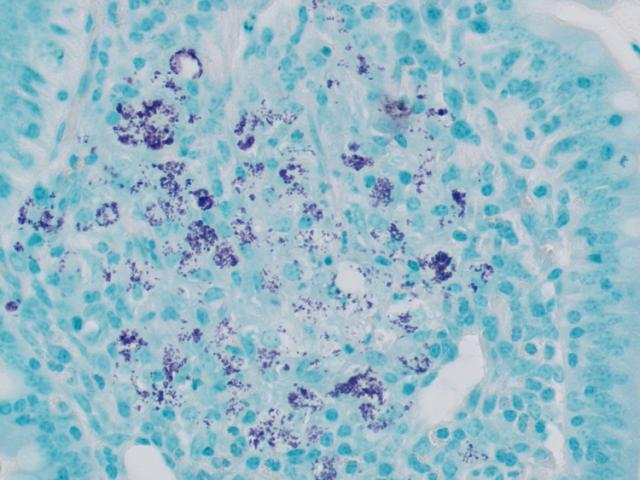
AFB Stain, Kinyoun
Reagents for this procedure are sold as individual stain solutions and are available for purchase under separate part numbers with storage requirements and expiration date designated per bottle.
SOLUTION:
| 250 ml | 500 ml | |
| Carbol Fuchsin Stain, Kinyoun | Part 1031A | Part 1031B |
Additionally Needed:
| Acid Fast Bacteria (AFB) Control Slides | Part 4011 |
| Acid Alcohol 1% | Part 10011 |
| Methylene Blue Stain 0.14%, Alcoholic | Part 12401 |
| Xylene, ACS | Part 1445 |
| Alcohol, Ethyl Denatured, 100% | Part 10841 |
| Alcohol, Ethyl Denatured, 95% | Part 10842 |
For storage requirements and expiration date refer to individual product labels.
APPLICATION:
Newcomer Supply Carbol Fuchsin Stain, Kinyoun is used in the Kinyoun AFB Stain to demonstrate the presence of acid-fast mycobacteria in tissue sections. Carbol Fuchsin Stain, Kinyoun is a concentrated phenol and basic fuchsin solution that works to permeate the lipoid capsule of acid-fast organisms, rendering them resistant to acid alcohol decolorization.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply stain procedures are designed to be used with Coplin jars filled to 40 ml following the provided staining procedure.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Filter Carbol Fuchsin Stain, Kinyoun with filter paper before use.
STAINING PROCEDURE:
-
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
-
- See Procedure Notes #1 and #2.
-
- Stain in freshly filtered Carbol Fuchsin Stain, Kinyoun for 15 minutes at room temperature. Keep solution covered.
-
- See Procedure Note #3.
-
- Wash in running tap water for 2 to 3 minutes.
- Differentiate in Acid Alcohol 1% (Part 10011) until color no longer runs off the slide and sections are pale pink; 3 to 10 rapid dips.
- Wash in running tap water 3 to 5 minutes; rinse in distilled water.
- Counterstain in Methylene Blue Stain 0.14%, Alcoholic (Part 12401); 3-6 dips.
-
- See Procedure Note #4.
-
- Wash in running tap water for 1 minute; rinse in distilled water.
- Dehydrate quickly in two changes each of 95% and 100% ethyl alcohol. Clear in three changes of xylene, 10 dips each; coverslip with compatible mounting medium.
- Deparaffinize sections thoroughly in three changes of xylene, 3 minutes each. Hydrate through two changes each of 100% and 95% ethyl alcohols, 10 dips each. Wash well with distilled water.
RESULTS:
| Acid-fast bacteria | Bright red |
| Background | Pale blue |
PROCEDURE NOTES:
-
- Drain slides after each step to prevent solution carry over.
- Do not allow sections to dry out at any point during procedure.
- Sections can remain in Carbol Fuchsin Stain, Kinyoun for up to 60 minutes without adverse effect. Additional differentiation may be required in Step #6.
- If preferred, Light Green SF Yellowish Stain 0.1%, Aqueous (Part 12203) can be used as a counterstain in place of Methylene Blue.
-
- Stain for 3-6 dips.
- Rinse with one quick dip in distilled water or proceed directly to Step #10 without a distilled water rinse.
-
- If using a xylene substitute, closely follow the manufacturer’s recommendations for deparaffinization and clearing steps.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik. Histotechnology: A Self-Instructional Text. 3rd ed. Chicago, Ill.: American Society of Clinical Pathologists, 2009. 224-226.
- J.J. “A Note on Uhlenhuths Method for Sputum Examination, for Tubercle Bacilli.” American Journal of Public Health 5.9 (1915). 867-870.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 236-237.
- Modifications developed by Newcomer Supply Laboratory.