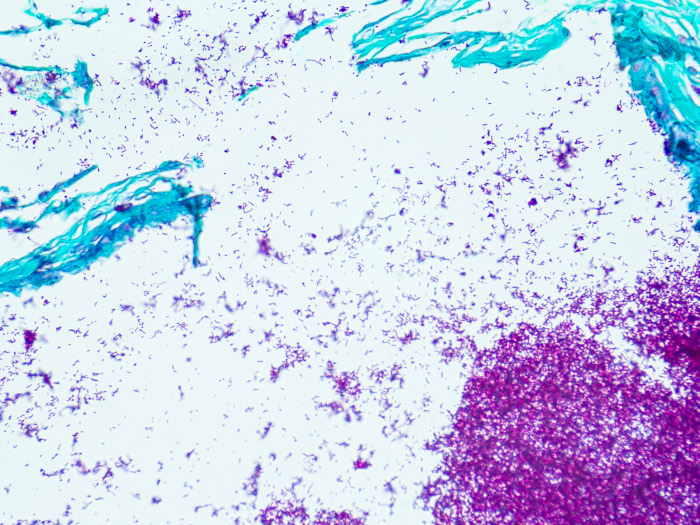
AFB, Fite Stain Kit
Detects the presence of either Nocardia sp. or Mycobacterium leprae sp. (causative agent of leprosy) in tissue sections.
AFB, FITE STAIN KIT INCLUDES:
| Part 91013A | ||
| Solution A: | Xylene/Peanut Oil, 2:1 | 500 ml |
| Solution B: | Carbol Fuchsin Stain, Ziehl-Neelsen | 250 ml |
| Solution C: | Acid Alcohol 1% | 250 ml |
| Solution D: | Light Green SF Yellowish Stain 0.1%, Aqueous | 250 ml |
COMPLIMENTARY POSITIVE CONTROL SLIDES: Enclosed are two complimentary unstained positive control slides for the initial verification of staining techniques and reagents. Verification must be documented by running one Newcomer Supply complimentary positive control slide along with your current positive control slide for the first run. Retain the second complimentary control slide for further troubleshooting, if needed.
Individual stain solutions and additional control slides may be available for purchase under separate part numbers.
Additionally Needed:
| Xylene, ACS | Part 1445 |
For storage requirements and expiration date refer to individual bottle labels.
APPLICATION:
Newcomer Supply AFB, Fite Stain Kit procedure is used to detect the presence of either Nocardia sp. or Mycobacterium leprae sp. (causative agent of leprosy) in tissue sections. Procedural variations are included for detection of either organism.
METHOD:
Fixation: Formalin 10%, Phosphate Buffered (Part 1090)
Technique: Paraffin sections cut at 4 microns
Solutions: All solutions are manufactured by Newcomer Supply, Inc.
All Newcomer Supply Stain Kits are designed to be used with Coplin jars filled to 40 ml following the staining procedure provided below. Some solutions in the kit may contain extra volumes.
PRESTAINING PREPARATION:
-
- If necessary, heat dry tissue sections/slides in oven.
- Filter Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen with high quality filter paper.
- If staining for Nocardia sp., prepare Diluted Acid Alcohol Solution:
-
- Solution C: Acid Alcohol 1% 20 ml
- Distilled water 20 ml
-
STAINING PROCEDURE:
-
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 10 minutes each.
-
- See Procedure Note #1
-
- Drain slides, wipe off excess oil, and blot to opacity taking care to remove residual oil.
-
- See Procedure Note #2.
-
- Stain in freshly filtered Solution B: Carbol Fuchsin Stain, Ziehl-Neelsen for 15 minutes at room temperature.
- Rinse well in distilled water.
- Differentiation for Nocardia sp.:
-
- Differentiate slides individually in Diluted Acid Alcohol Solution (Step #3) until background is pale pink; 10-20 dips.
- Quickly rinse in distilled water and check microscopically for correct differentiation.
-
- Differentiation for Mycobacterium leprae sp.:
-
- Differentiate slides individually in Solution C: Acid Alcohol 1% until sections are light pink; 5-10 dips.
-
- Rinse well in distilled water.
- Counterstain in Solution D: Light Green SF Yellowish Stain 0.1%, Aqueous; 5-10 dips.
- Rinse in distilled water.
- Blot excess water from slide and air-dry or oven-dry completely.
- Dip dried slides in xylene; coverslip with compatible mounting medium.
- Deparaffinize slides in Solution A: Xylene/Peanut Oil, 2:1, two changes for 10 minutes each.
RESULTS:
| Acid-fast bacilli and Mycobacterium leprae sp. | Red |
| Nocardia sp. | Red |
| Other tissue elements | Green |
PROCEDURE NOTES:
-
- Acid-fastness of leprosy organisms is enhanced when the waxy capsule is protected by the mixture of xylene/peanut oil and avoidance of dehydrating solutions.
- It is important to blot well, residual oil may produce staining artifact.
- A small percentage of Nocardia sp. organisms may resist taking the red stain and stain green due to the growth phase of the individual organism.
- If using a xylene substitute, closely follow the manufacturer’s recommendations for coverslipping step.
REFERENCES:
-
- Carson, Freida L., and Christa Hladik Cappellano. Histotechnology: A Self-instructional Text. 4th ed. Chicago: ASCP Press, 2015. 220-221.
- Fite, George, P.J. Cambre and M.H. Turner. “Procedure for Demonstrating Lepra Bacilli in Paraffin Sections”. Archives of Pathology 43 (1947). 624-625.
- Sheehan, Dezna C., and Barbara B. Hrapchak. Theory and Practice of Histotechnology. 2nd ed. St. Louis: Mosby, 1980. 237.
- Modifications developed by Newcomer Supply Laboratory.